Details of the Drug
General Information of Drug (ID: DMQP5I3)
| Drug Name |
Lacidipine
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Lacidipine; Lacidipine (Lacipil, Motens); Lacidipino; Lacidipino [Spanish]; Lacidipinum [Latin]; Lacimen; Lacipil; Motens; trans Lacidipine; 103890-78-4; 260080034N; 4-(o-((E)-2-Carboxyvinyl)phenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid, 4-tert-butyl diethyl ester; C26H33NO6; DSSTox_CID_26429; DSSTox_GSID_46429; DSSTox_RID_81607; GR 43659 X; GR 43659X; GR-43659X; GX-1048; UNII-260080034N; diethyl 2,6-dimethyl-4-[2-[(E)-3-[(2-methylpropan-2-yl)oxy]-3-oxoprop-1-enyl]phenyl]-1,4-dihydropyridine-3,5-dicarboxylate
|
|||||
| ATC Code | ||||||
| Structure |
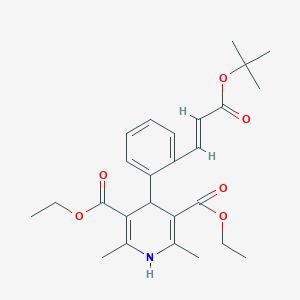 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 455.5 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | |||||
| Rotatable Bond Count (rotbonds) | 11 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
