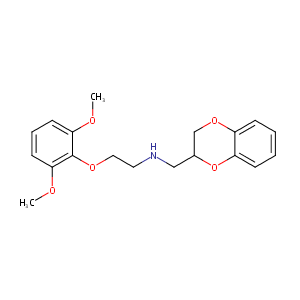
| Synonyms |
WB-4101; WB4101; WB 4101; (2-(2',6'-Dimethoxy)phenoxyethylamino)methylbenzo-1,4-dioxane; (2-(2',6'-Dimethoxy)phenoxyethylamino)methylbenzodioxan; 613-67-2; CHEMBL25554; CHEBI:64098; N-(2-(2,6-Dimethoxyphenoxy)ethyl)-2,3-dihydro-1,4-benzodioxin-2-methanamine; 1,4-Benzodioxin-2-methanamine, N-(2-(2,6-dimethoxyphenoxy)ethyl)-2,3-dihydro-; N-(2,3-dihydro-1,4-benzodioxin-2-ylmethyl)-2-(2,6-dimethoxyphenoxy)ethanamine; 1,4-benzodioxin-2-methanamine, n-[2-(2,6-dimethoxyphenoxy)ethyl]-2,3-dihydro-; MLS000859914
|
| Chemical Identifiers |
- Formula
- C19H23NO5
- IUPAC Name
N-(2,3-dihydro-1,4-benzodioxin-3-ylmethyl)-2-(2,6-dimethoxyphenoxy)ethanamine - Canonical SMILES
-
COC1=C(C(=CC=C1)OC)OCCNCC2COC3=CC=CC=C3O2
- InChI
-
InChI=1S/C19H23NO5/c1-21-17-8-5-9-18(22-2)19(17)23-11-10-20-12-14-13-24-15-6-3-4-7-16(15)25-14/h3-9,14,20H,10-13H2,1-2H3
- InChIKey
-
GYSZUJHYXCZAKI-UHFFFAOYSA-N
|