Details of the Drug
General Information of Drug (ID: DMR2J95)
| Drug Name |
Omadacycline
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | UNII-090IP5RV8F; 090IP5RV8F; Omadacycline [USAN:INN]; Omadacycline (USAN); AC1NUY3H; Amadacycline methanesulfonate; SCHEMBL152 | ||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Enterobacter cloacaeStreptococcus pyogenesStreptococcus pneumoniaeStaphylococcus aureusHaemophilus influenzaeHaemophilus parainfluenzaeKlebsiella pneumoniaeLegionella pneumophilaMycoplasma pneumoniaeEnterococcus faecalisStaphylococcus lugdunensisStreptococcus anginosusStreptococcus constellatusStreptococcus intermediusChlamydophila pneumoniae
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Structure |
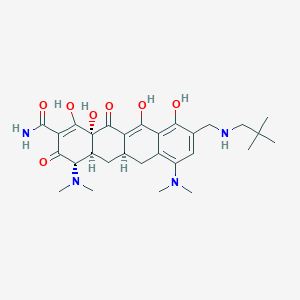 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 556.6 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 6 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Omadacycline Enters the Ring: A New Antimicrobial Contender.Pharmacotherapy. 2018 Dec;38(12):1194-1204. | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT00865280) Study the Safety and Efficacy of PTK 0796 in Patients With Complicated Skin and Skin Structure Infection (CSSSI). U.S. National Institutes of Health. | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB12455) | ||||
| 5 | Clinical disposition, metabolism and in vitro drug-drug interaction properties of omadacycline. Xenobiotica. 2017 Aug;47(8):682-696. doi: 10.1080/00498254.2016.1213465. Epub 2016 Aug 8. | ||||
| 6 | In Vitro and In Vivo Assessments of Cardiovascular Effects with Omadacycline. Antimicrob Agents Chemother. 2016 Aug 22;60(9):5247-53. doi: 10.1128/AAC.00320-16. Print 2016 Sep. | ||||
