Details of the Drug
General Information of Drug (ID: DMRMDWA)
| Drug Name |
Estropipate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Estropipate; 7280-37-7; Piperazine estrone sulfate; Harmogen; Ogen; Ortho-Est; UNII-SVI38UY019; Piperazine estronesulfate; Sulestrex; SVI38UY019; Estra-1,3,5(10)-trien-17-one, 3-(sulfooxy)-, compd. with piperazine (1:1); OGEN 2.5; Estropipate (USP); OGEN 5; Ogen (TN); OGEN .625; OGEN 1.25; EINECS 230-696-3; piperazine (8R,9S,13S,14S)-13-methyl-17-oxo-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3-yl sulfate; Piperazine oestrone sulphate; Conjugated estrogens: piperazine estrone sulfate; Estrone hydrogen sulf
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
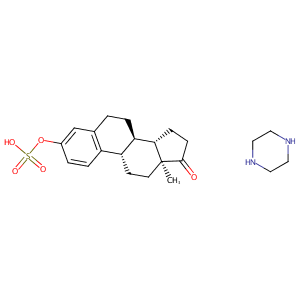 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 436.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hypogonadism | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A61.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | US patent application no. 5,004,651, Stabilizing system for solid dosage forms. | ||||
| 3 | Estrogen regulation of the cytochrome P450 3A subfamily in humans. J Pharmacol Exp Ther. 2004 Nov;311(2):728-35. | ||||
