Details of the Drug
General Information of Drug (ID: DMSPN58)
| Drug Name |
Clomethiazole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Clomethiazole; 533-45-9; Chlormethiazole; 5-(2-CHLOROETHYL)-4-METHYLTHIAZOLE; Distraneurin; Emineurina; Clomethiazolum; Chlormethiazol; Chlorethiazol; Chlorethiazole; Somnevrin; Clometiazole; Clomethiazol; 5-(2-Chloroethyl)-4-methyl-1,3-thiazole; Thiazole, 5-(2-chloroethyl)-4-methyl-; Clometiazolo [DCIT]; Chloro-S.C.T.Z.; C6H8ClNS; UNII-0C5DBZ19HV; Clometiazol [INN-Spanish]; Clomethiazolum [INN-Latin]; WY 1485; Clomethiazole [INN]; SCTZ [as edisylate]; EINECS 208-565-7; 4-Methyl-5-(beta-chloroethyl)thiazole; BRN 0114244; 0C5DBZ19HV
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
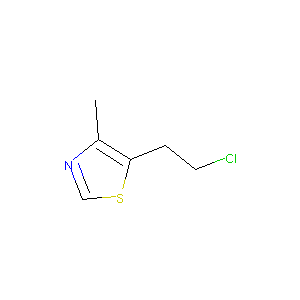 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 161.65 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
References
