Details of the Drug
General Information of Drug (ID: DMTYBC9)
| Drug Name |
OXAMATE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Oxamic acid; OXAMIC ACID; 471-47-6; 2-Amino-2-oxoacetic acid; oxamate; Acetic acid, aminooxo-; Oxalamic acid; Oxamidic acid; Oxalic acid monoamide; amino(oxo)acetic acid; Aminooxoacetic acid; Glycine, 2-oxo-; Oxamate (repellent); Glyoxylic acid, amino-; Formic acid, carbamoyl-; UNII-QU60N5OPLG; Oxamate, (aminocarbonyl)-; carbamoylformic acid; Formic acid, (aminocarbonyl)-; QU60N5OPLG; Oxamate (insect repellant); Acetic acid, 2-amino-2-oxo-; CHEBI:18058; Oxamic acid, 98%; OXM; C2H3NO3; Oxalic monoamide; Oxamate, 3; EINECS 207-443-0; NSC 47001; Oxamic Acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
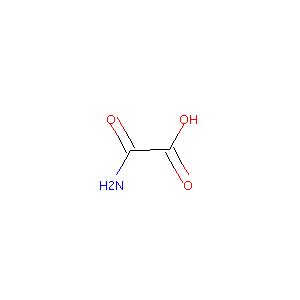 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 89.05 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
