Details of the Drug
General Information of Drug (ID: DMVJS2K)
| Drug Name |
Monobenzone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Agerite; Alba; Benoquin; Benzoquin; Carmifal; Depigman; Dermochinona; Leucodinine; Monobenzon; Monobenzona; Monobenzonum; PBP; Pigmex; Superlite; Agerite alba; Benzyl hydroquinone; Hydrochinon monobenzylether; Hydrochinon monobenzylether [Czech]; Hydroquinone benzyl ether; Hydroquinone monobenzyl ether; ICN brand of monobenzone; Monobenzone [INN]; Monobenzyl Ether of Hydroquinone; Monobenzyl ether hydroquinone; Monobenzyl hydroquinone; Alba-Dome; Benoquin (TN); Benoquin, Monobenzone; Benzyl p-hydroxyphenyl ether; Carmifal(TN); Depigman (TN); Dermochinona (TN); HYDROQUINONE MONOBENZYL ETHER, N F; Leucodinine (TN); Monobenzon (TN); Monobenzona [INN-Spanish]; Monobenzonum [INN-Latin]; Novo-depigman; P-BENZYLOXYPHENOL; P-Hydroxyphenyl benzyl ether; Pigmex (TN); Superlite (TN); Superlite (antioxidant); Monobenzone (USP/INN); P-(BENZYLOXY)PHENOL; 4-(Benzyl-Oxy)Phenol; 4-(Benzyloxy)phenol; 4-(Benzyloxyl)phenol; 4-(Phenylmethoxy)phenol; 4-Benzyloxyphenol; 4-benzyloxy phenol; 4-phenylmethoxy-phenol; 4-phenylmethoxyphenol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Dermatologic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
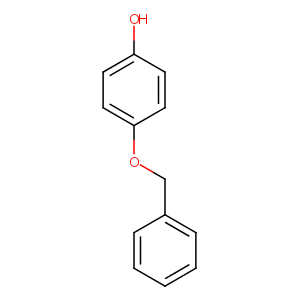 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 200.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Vitiligo | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ED63.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
