Details of the Drug
General Information of Drug (ID: DMVXLOD)
| Drug Name |
IMRECOXIB
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Imrecoxib; UNII-SGW6W5758V; SGW6W5758V; CHEMBL504535; 395683-14-4; Imricoxib; BAP-909; SCHEMBL3034253; BDBM50293282; AKOS032954049; DB12354; LS-194053; 2H-Pyrrol-2-one, 1,5-dihydro-3-(4-methylphenyl)-4-(4-(methylsulfonyl)phenyl)-1-propyl-; BAP-909; ;
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
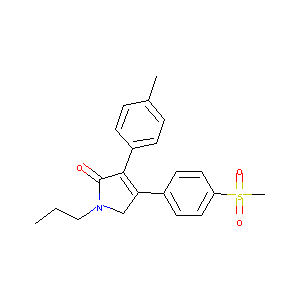 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 369.5 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | |||||
| Rotatable Bond Count (rotbonds) | 5 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
References
