Details of the Drug
General Information of Drug (ID: DMWG706)
| Drug Name |
4-(2-aminopyrimidin-4-ylamino)benzenesulfonamide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NSC135784; 2153-13-1; 4-[(2-aminopyrimidin-4-yl)amino]benzenesulfonamide; UNII-1DW8U29X8S; CHEMBL6633; 1DW8U29X8S; 4-((2-amino-4-pyrimidinyl)amino)benzenesulfonamide; NSC-135784; NSC683526; AC1L1KSM; NCIStruc2_000899; NCIStruc1_000852; Oprea1_770349; SCHEMBL3791868; ZINC56465; CTK4E7061; BDBM10873; NCI135784; NCGC00014345; CCG-38281; AKOS030546962; aromatic/heteroaromatic sulfonamide 18; MCULE-1716829198; NCGC00097454-01; NCGC00014345-02; NCI60_000801; EN300-24853; 4-(2-Amino-4-pyrimidinylamino)benzenesulfonamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
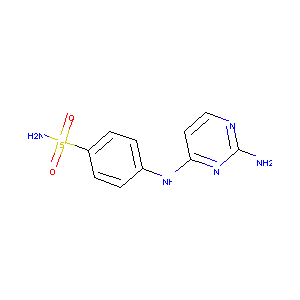 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 265.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
References
