Details of the Drug
General Information of Drug (ID: DMWIB0V)
| Drug Name |
Ethyl 7-methoxy-2-oxo-2H-chromene-3-carboxylate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ethyl 7-methoxy-2-oxo-2H-chromene-3-carboxylate; 6093-72-7; CHEMBL568385; ethyl 7-methoxy-2-oxochromene-3-carboxylate; 7-Methoxy-2-oxo-2H-chromene-3-carboxylic acid ethyl ester; MLS000554938; AC1LIZL3; Oprea1_386697; AC1Q645E; SCHEMBL1406462; CTK8D4125; CHEBI:108321; MolPort-000-258-049; ZINC499358; HMS2332G10; STK527622; BDBM50303495; AKOS002230558; MCULE-3724351908; AJ-23200; SMR000147055; TC-069558; KB-296861; AX8285479; ST50114633; 2-Oxo-7-methoxy-2H-1-benzopyran-3-carboxylic acid ethyl ester; 2H-1-Benzopyran-3-carboxylic acid,
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
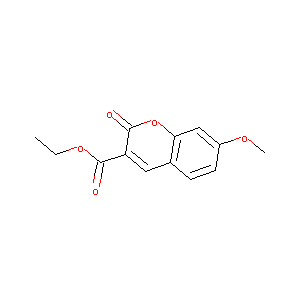 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 248.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
