Details of the Drug
General Information of Drug (ID: DMWU682)
| Drug Name |
PX-102
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Px-102; PX20606 trans-isomer; UNII-378SU5NO8S; 378SU5NO8S; CHEMBL3822773; 1268244-85-4 (trans-isomer); 4-((1S,2S)-2-(2-CHLORO-4-((5-CYCLOPROPYL-3-(2,6-DICHLOROPHENYL)ISOXAZOL-4-YL)METHOXY)PHENYL)CYCLOPROPYL)BENZOIC ACID; Px-104; 2020096-17-5; 1268244-85-4; PX 20606; SCHEMBL17087854; 4-(2-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-1,2-oxazol-4-yl)methoxy)phenyl)cyclopropyl)benzoic acid; BDBM50185707; ZINC115372389; DB15416; 4-(2-(2-Chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-4-isoxazolyl)methoxy)phenyl)cyclopropyl)benzoic acid; AC-30349; PX-20606; PX-102(PX-20606); UNII-6TU6SUZ3BY component XBUXXJUEBFDQHD-NHCUHLMSSA-N; Benzoic acid, 4-((1R,2R)-2-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-4-isoxazolyl)methoxy)phenyl)cyclopropyl)-, rel-(-)-; Benzoic acid, 4-(2-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)-4-isoxazolyl)methoxy)phenyl)cyclopropyl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
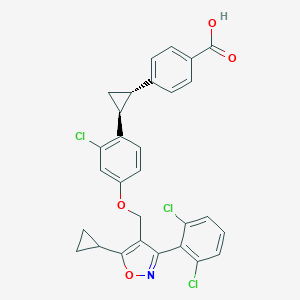 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 554.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hepatic fibrosis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | DB93.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
