Details of the Drug
General Information of Drug (ID: DMWX3HT)
| Drug Name |
ACEA
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Arachidonyl-2-chloroethylamide; arachidonyl-2-chloroethylamide; Arachidonyl-2'-chloroethylamide; CHEMBL151167; (5Z,8Z,11Z,14Z)-N-(2-chloroethyl)icosa-5,8,11,14-tetraenamide; C22H36ClNO; Arachidonoyl-2-chloroethylamide; AC1NSJTE; GTPL738; SCHEMBL1517676; MolPort-003-940-362; HMS3649N11; MFCD02683581; BN0049; BDBM50072769; ZINC13675373; 1595AH; AKOS024456526; Arachidonyl-2'-chloroethylamide hydrate; NCGC00162406-01; LS-63778; ACM273734079; SR-01000946682; L000112; SR-01000946682-1; N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
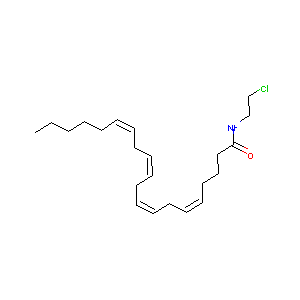 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 366 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 16 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
