Details of the Drug
General Information of Drug (ID: DMYNJHI)
| Drug Name |
ARC029
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nilvadipine; nilvadipine; 75530-68-6; Escor; Nivadil; Nivadipine; FR-34235; Nilvadipinum [Latin]; Nilvadipino [Spanish]; FR 34235; FK 235; Nilvadipine [USAN:INN:JAN]; Nilvadipine (ARC029); FK-235; F-102362; BRN 3572609; F 102,362; CL-287389; CL 287,389; FAIIFDPAEUKBEP-UHFFFAOYSA-N; C19H19N3O6; NCGC00167435-01; 5-Isopropyl 3-methyl 2-cyano-1,4-dihydro-6-methyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate; 3,5-Pyridinedicarboxylic acid, 2-cyano-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-, 3-methyl 5-(1-methylethyl) ester; Nilvadipino; NILVADIPINE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
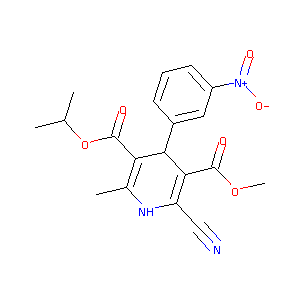 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 385.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
