Details of the Drug
General Information of Drug (ID: DMYX7NI)
| Drug Name |
Acetohydroxamic Acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AHA; Acethydroxamsaeure; Acethydroxamsaure; Acetohydroxamate; HAE; Lithostat; Acethydroxamic acid; Acethydroxamsaeure [German]; Acetohydroximic acid; Acetyl hydroxyamino; Acetylhydroxamic acid; Acide acetohydroxamique; Acide acetohydroxamique [French]; Acido acetohidroxamico; Acido acetohidroxamico [Spanish]; Acidum acetohydroxamicum; Acidum acetohydroxamicum [Latin]; Cetohyroxamic acid; Methylhydroxamic acid; SJX HLdmMAH; AHA (TN); Acetic acid, oxime; Acetohydroxamic acid [USAN:INN]; Lithostat (TN); N-Acetyl hydroxyacetamide; N-Acetylhydroxylamine; N-Hydroxyacetamide; N-hydroxyacetimidic acid; N-hydroxyethanimidic acid; S14-0751; Acetohydroxamic acid (USP/INN); Acetamide, N-hydroxy-(9CI)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiinfective Agents
|
||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
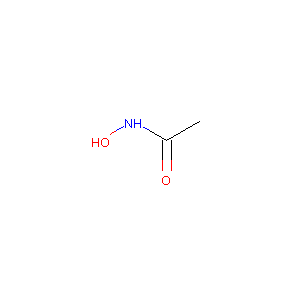 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 75.07 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Acetohydroxamic Acid (Comorbidity)
|
|||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 018749. | ||||
|---|---|---|---|---|---|
| 2 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Enzymatic, immunological and phylogenetic characterization of Brucella suis urease. BMC Microbiol. 2008 Jul 19;8:121. | ||||
| 5 | New bispyridinium oximes: in vitro and in vivo evaluation of their biological efficiency in soman and tabun poisoning. Chem Biol Interact. 2008 Sep 25;175(1-3):413-6. | ||||
| 6 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 7 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
