Details of the Drug
General Information of Drug (ID: DMZUAJ4)
| Drug Name |
PMX-53
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
YOKBGCTZYPOSQM-HPSWDUTRSA-N; PMX53; CHEMBL41547; (2S)-2-acetamido-N-[(3S,9S,12S,15R,18S)-15-(cyclohexylmethyl)-9-[3-(diaminomethylideneamino)propyl]-12-(1H-indol-3-ylmethyl)-2,8,11,14,17-pentaoxo-1,7,10,13,16-pentazabicyclo[1630]henicosan-3-yl]-3-phenylpropanamide; PMX 53; AcF-[OP(D-Cha)WR]; Ac-Phe-[Orn-Pro-cha-Trp-Arg]; C5aR-AP; AC1OCFH0; GTPL579; SCHEMBL16492460; SCHEMBL12971688; 219639-75-5; BDBM50111445; AcF-[OP(D-Cha)WR]; N-Acetyl-L-phenylalanyl-L-ornithyl-L-prolyl-3-cyclohexyl-D-alanyl-L-tryptophyl-D-arginine N-52-C-16-lactam
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
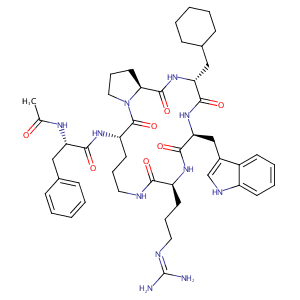 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 896.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 13 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Atopic dermatitis | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | EA80 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
