Details of the Drug
General Information of Drug (ID: DMZUR4N)
| Drug Name |
Triclosan
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
triclosan; 3380-34-5; 5-CHLORO-2-(2,4-DICHLOROPHENOXY)PHENOL; 2,4,4'-Trichloro-2'-hydroxydiphenyl ether; Irgasan; Cloxifenolum; Triclosanum; Irgasan DP300; Stri-Dex Face Wash; Phenol, 5-chloro-2-(2,4-dichlorophenoxy)-; Lexol 300; Stri-Dex Cleansing Bar; 5-Chloro-2-(2,4-dichloro-phenoxy)-phenol; Triclosanum [INN-Latin]; CH 3565; DP-300; Caswell No 186A; C12H7Cl3O2; UNII-4NM5039Y5X; HSDB 7194; CHEBI:164200; Ether, 2'-hydroxy-2,4,4'-trichlorodiphenyl; Phenyl ether, 2'-hydroxy-2,4,4'-trichloro-; EINECS 222-182-2; CHEMBL849; EPA; Aquasept; Cliniclean; Cloxifenol; Manusept; Sapoderm; TCL; Trisan; Clearasil DailyFace Wash; Dermtek Brand of Triclosan; GlaxoSmithKline Brand of Triclosan; Microshield T; Oxy Skin Wash; Pharmachem Brand of Triclosan; Reckitt Brand of Triclosan; SSL Brand of Triclosan; Ster Zac Bath Concentrate; SterZac Bath Concentrate; Trans Canaderm Brand of Triclosan; Triclosan Pharmachem Brand; Triclosan Reckitt Brand; IN1424; Irgasan DP 300; CH-3565; Johnson & Johnson Brand of Triclosan; Procter & Gamble Brand of Triclosan; Ster-Zac Bath Concentrate; Stri-Dex cleansing bar (TN); Triclosan (USP/INN); Triclosan [USAN:BAN:INN]; 2,4,4'-Trichloro-2'-hydroxy diphenyl ether; 2-Hydroxy-2',4,4'-trichlorodiphenyl Ether; WL-1001
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||||||||||
| Affected Organisms |
BacteriaFungi, yeast and protozoans
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
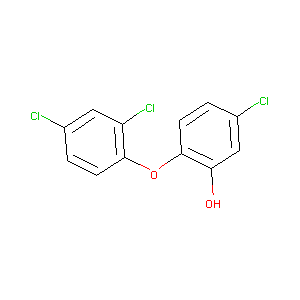 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 289.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
