Details of the Drug
General Information of Drug (ID: DMZW9HA)
| Drug Name |
Olsalazine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Azodisal; Dipentum; Olsalazine sodium; Di-mesalazine | ||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
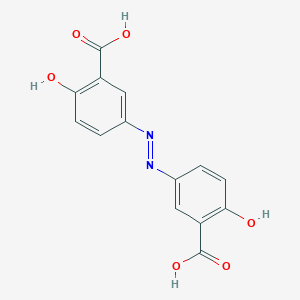 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 302.24 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Olsalazine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | ClinicalTrials.gov (NCT00004288) Phase II Pilot Study of Olsalazine for Ankylosing Spondylitis. U.S. National Institutes of Health. | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 4 | Reaction mechanism of azoreductases suggests convergent evolution with quinone oxidoreductases. Protein Cell. 2010 Aug;1(8):780-90. | ||||
| 5 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 6 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 7 | Wong GT, Lee EY, Irwin MG. Contrast induced nephropathy in vascular surgery.?Br J Anaesth. 2016;117 Suppl 2:ii63-ii73. [PMID: 27566809] | ||||
| 8 | Novis BH, Korzets Z, Chen P, Bernheim J "Nephrotic syndrome after treatment with 5-aminosalicylic acid." Br Med J (Clin Res Ed) 296 (1988): 1442. [PMID: 3132281] | ||||
| 9 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
