Details of the Drug
General Information of Drug (ID: DMKMBES)
| Drug Name |
Plazomicin
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | ACHN-490; UNII-LYO9XZ250J; 1154757-24-0; LYO9XZ250J; Plazomicin [USAN:INN]; Plazomicin (USAN); ZINC68150640; DB12615; D10151; D-Streptamine, | ||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Klebsiella pneumoniaeEscherichia coliEnterobacterAcinetobacterPseudomonas aeruginosa
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
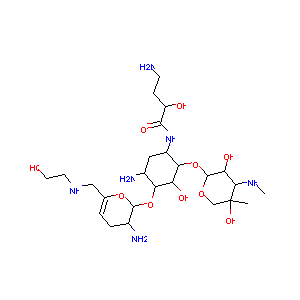 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 592.7 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -6.2 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 13 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 11 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 15 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Plazomicin (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | In vitro activity of plazomicin against -lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE).J Antimicrob Chemother. 2017 Oct 1;72(10):2792-2795. | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT01970371) A Study of Plazomicin Compared With Colistin in Patients With Infection Due to Carbapenem-Resistant Enterobacteriaceae (CRE). U.S. National Institutes of Health. | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis. 2018 Jun 8;4(6):980-987. | ||||
| 5 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 6 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 7 | Burkett L, Bikhazi GB, Thomas KC Jr, Rosenthal DA, Wirta MG, Foldes FF "Mutual potentiation of the neuromuscular effects of antibiotics and relaxants." Anesth Analg 58 (1979): 107-15. [PMID: 571233] | ||||
| 8 | Farag MM, Mikhail MR, Abdel-Meguid E, Abdel-Tawab S "Assessment of gentamicin-induced nephrotoxicity in rats treated with low doses of ibuprofen and diclofenac sodium." Clin Sci 91 (1996): 187-91. [PMID: 8795442] | ||||
