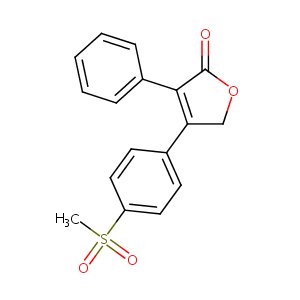
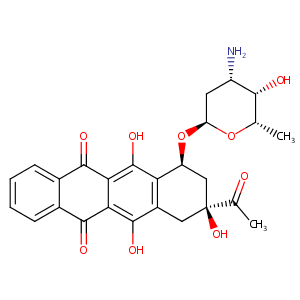
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Cytochrome P450 1A2 (CYP1A2)
|
OTLLBX48
|
CP1A2_HUMAN
|
Decreases Activity
|
[11] |
|
Prostaglandin G/H synthase 1 (PTGS1)
|
OTHCRLEC
|
PGH1_HUMAN
|
Decreases Expression
|
[12] |
|
Prostaglandin G/H synthase 2 (PTGS2)
|
OT75U9M4
|
PGH2_HUMAN
|
Increases Expression
|
[13] |
|
Stearoyl-CoA desaturase (SCD)
|
OTB1073G
|
SCD_HUMAN
|
Increases Expression
|
[5] |
|
Suppressor of cytokine signaling 3 (SOCS3)
|
OTY183WJ
|
SOCS3_HUMAN
|
Increases Expression
|
[5] |
|
Ephrin type-B receptor 6 (EPHB6)
|
OTKNN6GC
|
EPHB6_HUMAN
|
Decreases Expression
|
[5] |
|
Polyunsaturated fatty acid lipoxygenase ALOX15B (ALOX15B)
|
OTWQQ08W
|
LX15B_HUMAN
|
Affects Expression
|
[5] |
|
Tetraspanin-2 (TSPAN2)
|
OTTDPQF1
|
TSN2_HUMAN
|
Decreases Expression
|
[5] |
|
SH2 domain-containing protein 1A (SH2D1A)
|
OTLU49I5
|
SH21A_HUMAN
|
Decreases Expression
|
[5] |
|
Filamin-B (FLNB)
|
OTPCOYL6
|
FLNB_HUMAN
|
Increases Expression
|
[5] |
|
Annexin A9 (ANXA9)
|
OTKNYMU2
|
ANXA9_HUMAN
|
Decreases Expression
|
[5] |
|
Metastasis-associated protein MTA2 (MTA2)
|
OTCCYIQJ
|
MTA2_HUMAN
|
Decreases Expression
|
[5] |
|
Neurofascin (NFASC)
|
OTBDUXZT
|
NFASC_HUMAN
|
Decreases Expression
|
[5] |
|
Angiopoietin-related protein 1 (ANGPTL1)
|
OTXIN6V5
|
ANGL1_HUMAN
|
Decreases Expression
|
[5] |
|
Integrin beta-like protein 1 (ITGBL1)
|
OTJDHE17
|
ITGBL_HUMAN
|
Affects Expression
|
[5] |
|
Alpha-1-antichymotrypsin (SERPINA3)
|
OT9BP2S0
|
AACT_HUMAN
|
Increases Expression
|
[5] |
|
Complement C3 (C3)
|
OTCH5GS0
|
CO3_HUMAN
|
Increases Expression
|
[5] |
|
Transferrin receptor protein 1 (TFRC)
|
OT8ZPBDL
|
TFR1_HUMAN
|
Affects Expression
|
[5] |
|
Annexin A1 (ANXA1)
|
OT5OFDJC
|
ANXA1_HUMAN
|
Affects Expression
|
[5] |
|
Superoxide dismutase , mitochondrial (SOD2)
|
OTIWXGZ9
|
SODM_HUMAN
|
Increases Expression
|
[5] |
|
Interleukin-6 (IL6)
|
OTUOSCCU
|
IL6_HUMAN
|
Increases Expression
|
[5] |
|
Growth-regulated alpha protein (CXCL1)
|
OT3WCTZV
|
GROA_HUMAN
|
Affects Expression
|
[5] |
|
Clusterin (CLU)
|
OTQGG0JM
|
CLUS_HUMAN
|
Increases Expression
|
[5] |
|
Annexin A3 (ANXA3)
|
OTDD8OI7
|
ANXA3_HUMAN
|
Increases Expression
|
[5] |
|
C-C motif chemokine 2 (CCL2)
|
OTAD2HEL
|
CCL2_HUMAN
|
Increases Expression
|
[5] |
|
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1)
|
OTPWI88U
|
CEAM1_HUMAN
|
Affects Expression
|
[5] |
|
Hepatocyte growth factor (HGF)
|
OTGHUA23
|
HGF_HUMAN
|
Decreases Expression
|
[5] |
|
Phospholipase A2, membrane associated (PLA2G2A)
|
OT1NY7BF
|
PA2GA_HUMAN
|
Affects Expression
|
[5] |
|
Fatty acid-binding protein, adipocyte (FABP4)
|
OT3DKFOU
|
FABP4_HUMAN
|
Decreases Expression
|
[5] |
|
15-hydroxyprostaglandin dehydrogenase (HPGD)
|
OTYZI6JB
|
PGDH_HUMAN
|
Decreases Expression
|
[5] |
|
NAD(P)H dehydrogenase 1 (NQO1)
|
OTZGGIVK
|
NQO1_HUMAN
|
Affects Expression
|
[5] |
|
Interleukin-1 receptor antagonist protein (IL1RN)
|
OT308CBE
|
IL1RA_HUMAN
|
Increases Expression
|
[5] |
|
Cadherin-2 (CDH2)
|
OTH0Y56P
|
CADH2_HUMAN
|
Affects Expression
|
[5] |
|
Atrial natriuretic peptide receptor 2 (NPR2)
|
OT4651SI
|
ANPRB_HUMAN
|
Decreases Expression
|
[5] |
|
Ras GTPase-activating protein 1 (RASA1)
|
OTZJ3LP4
|
RASA1_HUMAN
|
Affects Expression
|
[5] |
|
Myelin and lymphocyte protein (MAL)
|
OTBM30SW
|
MAL_HUMAN
|
Increases Expression
|
[5] |
|
Cadherin-3 (CDH3)
|
OTKNLQU7
|
CADH3_HUMAN
|
Increases Expression
|
[5] |
|
Trans-acting T-cell-specific transcription factor GATA-3 (GATA3)
|
OTGP7D5Y
|
GATA3_HUMAN
|
Decreases Expression
|
[5] |
|
Tenascin (TNC)
|
OTK4FSHR
|
TENA_HUMAN
|
Increases Expression
|
[5] |
|
Ephrin type-A receptor 3 (EPHA3)
|
OTZJF3ML
|
EPHA3_HUMAN
|
Decreases Expression
|
[5] |
|
Nitric oxide synthase 1 (NOS1)
|
OT7M8XVG
|
NOS1_HUMAN
|
Affects Expression
|
[5] |
|
Protein PML (PML)
|
OT6SM2GD
|
PML_HUMAN
|
Increases Expression
|
[5] |
|
Signal transducer and activator of transcription 3 (STAT3)
|
OTAAGKYZ
|
STAT3_HUMAN
|
Increases Expression
|
[5] |
|
ETS translocation variant 5 (ETV5)
|
OTE2OBM4
|
ETV5_HUMAN
|
Decreases Expression
|
[5] |
|
Phosphatidylinositol 4-kinase alpha (PI4KA)
|
OT2L2G6C
|
PI4KA_HUMAN
|
Decreases Expression
|
[5] |
|
Leukemia inhibitory factor receptor (LIFR)
|
OT36W9O5
|
LIFR_HUMAN
|
Decreases Expression
|
[5] |
|
Prostaglandin E2 receptor EP3 subtype (PTGER3)
|
OTQODQUN
|
PE2R3_HUMAN
|
Decreases Expression
|
[5] |
|
Homeobox protein MOX-2 (MEOX2)
|
OTKZCJCB
|
MEOX2_HUMAN
|
Decreases Expression
|
[5] |
|
Nuclear receptor ROR-gamma (RORC)
|
OTUBFRPC
|
RORG_HUMAN
|
Decreases Expression
|
[5] |
|
Cysteine-rich secretory protein 3 (CRISP3)
|
OTBSWMPL
|
CRIS3_HUMAN
|
Increases Expression
|
[5] |
|
Paired mesoderm homeobox protein 1 (PRRX1)
|
OTTZK5G8
|
PRRX1_HUMAN
|
Affects Expression
|
[5] |
|
Afadin (AFDN)
|
OTTRU341
|
AFAD_HUMAN
|
Affects Expression
|
[5] |
|
Fractalkine (CX3CL1)
|
OTPLGI0E
|
X3CL1_HUMAN
|
Affects Expression
|
[5] |
|
Insulinoma-associated protein 1 (INSM1)
|
OTG8RV8E
|
INSM1_HUMAN
|
Decreases Expression
|
[5] |
|
Collagen alpha-1(XIV) chain (COL14A1)
|
OTLNJ13O
|
COEA1_HUMAN
|
Decreases Expression
|
[5] |
|
MBT domain-containing protein 1 (MBTD1)
|
OT2P6943
|
MBTD1_HUMAN
|
Decreases Expression
|
[5] |
|
Aldehyde oxidase (AOX1)
|
OT2FZP6H
|
AOXA_HUMAN
|
Decreases Expression
|
[5] |
|
Myocyte-specific enhancer factor 2C (MEF2C)
|
OTZGF1Y5
|
MEF2C_HUMAN
|
Affects Expression
|
[5] |
|
Desmocollin-1 (DSC1)
|
OTNII6GZ
|
DSC1_HUMAN
|
Affects Expression
|
[5] |
|
Periostin (POSTN)
|
OTWP62UA
|
POSTN_HUMAN
|
Affects Expression
|
[5] |
|
Angiopoietin-1 (ANGPT1)
|
OTVZ1NG3
|
ANGP1_HUMAN
|
Decreases Expression
|
[5] |
|
Corneodesmosin (CDSN)
|
OTQW4HV6
|
CDSN_HUMAN
|
Affects Expression
|
[5] |
|
Microtubule-associated protein RP/EB family member 2 (MAPRE2)
|
OTAE3MX4
|
MARE2_HUMAN
|
Decreases Expression
|
[5] |
|
Transcriptional enhancer factor TEF-3 (TEAD4)
|
OTJS0T2B
|
TEAD4_HUMAN
|
Increases Expression
|
[5] |
|
Forkhead box protein D1 (FOXD1)
|
OT80PRHS
|
FOXD1_HUMAN
|
Decreases Expression
|
[5] |
|
Rho GTPase-activating protein 29 (ARHGAP29)
|
OT8JH4TY
|
RHG29_HUMAN
|
Decreases Expression
|
[5] |
|
Inactive N-acetylated-alpha-linked acidic dipeptidase-like protein 2 (NAALADL2)
|
OT2HOGPQ
|
NADL2_HUMAN
|
Decreases Expression
|
[5] |
|
Collectin-12 (COLEC12)
|
OTIQTK9G
|
COL12_HUMAN
|
Decreases Expression
|
[5] |
|
Adhesion G-protein coupled receptor F1 (ADGRF1)
|
OTRAOBYH
|
AGRF1_HUMAN
|
Affects Expression
|
[5] |
|
E3 ubiquitin-protein ligase RNF144B (RNF144B)
|
OTN7FCDE
|
R144B_HUMAN
|
Increases Expression
|
[5] |
|
Adhesion G-protein coupled receptor G6 (ADGRG6)
|
OTY2UBXO
|
AGRG6_HUMAN
|
Affects Expression
|
[5] |
|
Neuronal PAS domain-containing protein 3 (NPAS3)
|
OT8D1ILB
|
NPAS3_HUMAN
|
Increases Expression
|
[5] |
|
Voltage-dependent calcium channel subunit alpha-2/delta-3 (CACNA2D3)
|
OTEH9HGE
|
CA2D3_HUMAN
|
Decreases Expression
|
[5] |
|
LIM homeobox transcription factor 1-alpha (LMX1A)
|
OTEEYD5L
|
LMX1A_HUMAN
|
Increases Expression
|
[5] |
|
Secreted frizzled-related protein 3 (FRZB)
|
OTTO3DPY
|
SFRP3_HUMAN
|
Affects Expression
|
[5] |
|
Protein lifeguard 2 (FAIM2)
|
OT6QINVO
|
LFG2_HUMAN
|
Decreases Expression
|
[5] |
|
Transmembrane protease serine 4 (TMPRSS4)
|
OTCCGY2K
|
TMPS4_HUMAN
|
Increases Expression
|
[5] |
|
FAS-associated factor 1 (FAF1)
|
OTGAMLM4
|
FAF1_HUMAN
|
Affects Expression
|
[5] |
|
A disintegrin and metalloproteinase with thrombospondin motifs 8 (ADAMTS8)
|
OT2KFY1S
|
ATS8_HUMAN
|
Affects Expression
|
[5] |
|
Krueppel-like factor 12 (KLF12)
|
OTVH4KD4
|
KLF12_HUMAN
|
Affects Expression
|
[5] |
|
E3 ubiquitin-protein ligase TRIM17 (TRIM17)
|
OTUJZUOS
|
TRI17_HUMAN
|
Affects Expression
|
[5] |
|
Prostacyclin synthase (PTGIS)
|
OTT3RW6N
|
PTGIS_HUMAN
|
Decreases Expression
|
[12] |
|
Tissue-type plasminogen activator (PLAT)
|
OTQPDNAB
|
TPA_HUMAN
|
Increases Expression
|
[14] |
|
Tumor necrosis factor (TNF)
|
OT4IE164
|
TNFA_HUMAN
|
Decreases Expression
|
[15] |
|
Interleukin-1 alpha (IL1A)
|
OTPSGILV
|
IL1A_HUMAN
|
Decreases Expression
|
[16] |
|
Interstitial collagenase (MMP1)
|
OTI4I2V1
|
MMP1_HUMAN
|
Increases Expression
|
[14] |
|
Antileukoproteinase (SLPI)
|
OTUNFUU8
|
SLPI_HUMAN
|
Increases Expression
|
[16] |
|
Stromelysin-1 (MMP3)
|
OTGBI74Z
|
MMP3_HUMAN
|
Increases Expression
|
[14] |
|
Interleukin-8 (CXCL8)
|
OTS7T5VH
|
IL8_HUMAN
|
Increases Expression
|
[14] |
|
Protachykinin-1 (TAC1)
|
OTM842YW
|
TKN1_HUMAN
|
Increases Expression
|
[17] |
|
Metalloproteinase inhibitor 3 (TIMP3)
|
OTDGQAD1
|
TIMP3_HUMAN
|
Decreases Expression
|
[14] |
|
Major histocompatibility complex class I-related gene protein (MR1)
|
OTZU3XX7
|
HMR1_HUMAN
|
Affects Expression
|
[18] |
| ------------------------------------------------------------------------------------ |
|
|
|
|