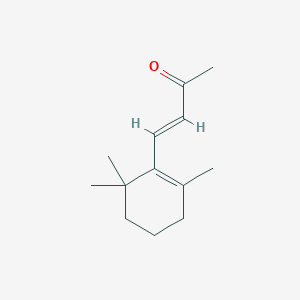
| Synonyms |
BETA-IONONE; beta-Cyclocitrylideneacetone; beta-E-Ionone; trans-beta-Ionone; (3E)-4-(2,6,6-trimethylcyclohex-1-en-1-yl)but-3-en-2-one; (E)-4-(2,6,6-Trimethylcyclohex-1-en-1-yl)but-3-en-2-one; (E)-beta-Ionone; .beta.-Ionone; 14901-07-6; 3-Buten-2-one, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-, (3E)-; 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one; 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one; 4-(2,6,6-Trimethylcyclohex-1-en-1-yl)but-3-en-2-one; 79-77-6; NSC 402758; UNII-A7NRR1HLH6
|
| Chemical Identifiers |
- Formula
- C13H20O
- IUPAC Name
(E)-4-(2,6,6-trimethylcyclohexen-1-yl)but-3-en-2-one - Canonical SMILES
-
CC1=C(C(CCC1)(C)C)C=CC(=O)C
- InChI
-
PSQYTAPXSHCGMF-BQYQJAHWSA-N
- InChIKey
-
1S/C13H20O/c1-10-6-5-9-13(3,4)12(10)8-7-11(2)14/h7-8H,5-6,9H2,1-4H3/b8-7+
|