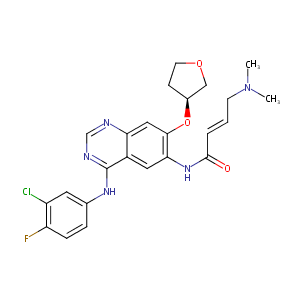
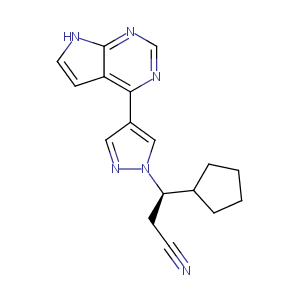
| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5667).
|
| 3 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5688).
|
| 5 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 6 |
Ruxolitinib FDA Label
|
| 7 |
Incyte begins Phase III trial of ruxolitinib to treat Covid-19. 20.April.2020.
|
| 8 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 9 |
Boehringer Ingelheim. Product Development Pipeline. June 2 2009.
|
| 10 |
Breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-gp/ABCB1) transport afatinib and restrict its oral availability and brain accumulation. Pharmacol Res. 2017 Jun;120:43-50.
|
| 11 |
Cysteine mapping in conformationally distinct kinase nucleotide binding sites: application to the design of selective covalent inhibitors. J Med Chem. 2011 Mar 10;54(5):1347-55. doi: 10.1021/jm101396q. Epub 2011 Feb 15.
|
| 12 |
Irreversible EGFR inhibitor EKB-569 targets low-LET -radiation-triggered rel orchestration and potentiates cell death in squamous cell carcinoma. PLoS One. 2011;6(12):e29705. doi: 10.1371/journal.pone.0029705. Epub 2011 Dec 29.
|
| 13 |
Tyrosine phosphorylation of the scaffold protein IQGAP1 in the MET pathway alters function. J Biol Chem. 2020 Dec 25;295(52):18105-18121. doi: 10.1074/jbc.RA120.015891. Epub 2020 Oct 21.
|
| 14 |
Oncoprotein SET dynamically regulates cellular stress response through nucleocytoplasmic transport in breast cancer. Cell Biol Toxicol. 2023 Aug;39(4):1795-1814. doi: 10.1007/s10565-022-09784-4. Epub 2022 Dec 19.
|
| 15 |
YM155 as an inhibitor of cancer stemness simultaneously inhibits autophosphorylation of epidermal growth factor receptor and G9a-mediated stemness in lung cancer cells. PLoS One. 2017 Aug 7;12(8):e0182149. doi: 10.1371/journal.pone.0182149. eCollection 2017.
|
| 16 |
Knockdown of CALM2 increases the sensitivity to afatinib in HER2-amplified gastric cancer cells by regulating the Akt/FoxO3a/Puma axis. Toxicol In Vitro. 2023 Mar;87:105531. doi: 10.1016/j.tiv.2022.105531. Epub 2022 Nov 29.
|
| 17 |
2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4.
|
| 18 |
Urokinase-type plasminogen activator receptor signaling is critical in nasopharyngeal carcinoma cell growth and metastasis.Cell Cycle. 2014;13(12):1958-69.
|
| 19 |
The Use of Anti-Inflammatory Drugs in the Treatment of People With Severe Coronavirus Disease 2019 (COVID-19): The Perspectives of Clinical Immunologists From China. Clin Immunol. 2020 May;214:108393.
|
| 20 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
|
|
|
|
|
|