| 1 |
ClinicalTrials.gov (NCT02412631) Addressing Post Cessation Weight Gain
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2941).
|
| 3 |
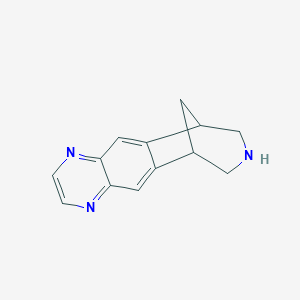
Varenicline FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5459).
|
| 5 |
Anti-obesity drugs. Expert Opin Pharmacother. 2008 Jun;9(8):1339-50.
|
| 6 |
Identification of human cytochrome P450 and flavin-containing monooxygenase enzymes involved in the metabolism of lorcaserin, a novel selective human 5-hydroxytryptamine 2C agonist. Drug Metab Dispos. 2012 Apr;40(4):761-71.
|
| 7 |
2006 drug approvals: finding the niche. Nat Rev Drug Discov. 2007 Feb;6(2):99-101.
|
| 8 |
Effect of human renal cationic transporter inhibition on the pharmacokinetics of varenicline, a new therapy for smoking cessation: an in vitro-in vivo study. Clin Pharmacol Ther. 2008 Apr;83(4):567-76.
|
| 9 |
Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42.
|
| 10 |
ClinicalTrials.gov (NCT02393547) Lorcaserin for Preventing Weight Gain Among Smokers
|
|
|
|
|
|
|