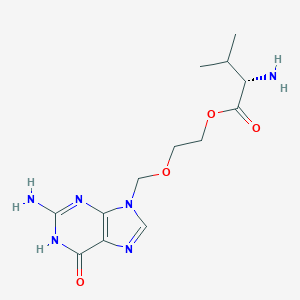
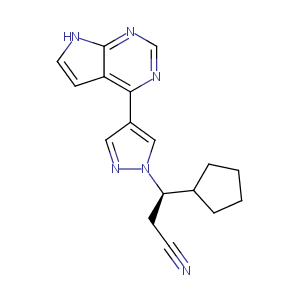
| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
Interventions for men and women with their first episode of genital herpes. Cochrane Database Syst Rev. 2016 Aug 30;2016(8):CD010684.
|
| 3 |
Interventions for prevention of herpes simplex labialis (cold sores on the lips). Cochrane Database Syst Rev. 2015 Aug 7;2015(8):CD010095.
|
| 4 |
Chickenpox. BMJ Clin Evid. 2011 Apr 11;2011:0912.
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4824).
|
| 6 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5688).
|
| 8 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 9 |
Ruxolitinib FDA Label
|
| 10 |
Incyte begins Phase III trial of ruxolitinib to treat Covid-19. 20.April.2020.
|
| 11 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 12 |
Extensive oral shedding of human herpesvirus 8 in a renal allograft recipient. Oral Microbiol Immunol. 2009 Apr;24(2):109-15.
|
| 13 |
Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998 May 19;246(2):470-5.
|
| 14 |
Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther. 2002 Mar;300(3):918-24.
|
| 15 |
Transport of amino acid-based prodrugs by the Na+- and Cl(-) -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004 Mar;308(3):1138-47.
|
| 16 |
Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167.
|
| 17 |
2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4.
|
| 18 |
Urokinase-type plasminogen activator receptor signaling is critical in nasopharyngeal carcinoma cell growth and metastasis.Cell Cycle. 2014;13(12):1958-69.
|
| 19 |
The Use of Anti-Inflammatory Drugs in the Treatment of People With Severe Coronavirus Disease 2019 (COVID-19): The Perspectives of Clinical Immunologists From China. Clin Immunol. 2020 May;214:108393.
|
| 20 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
|
|
|
|
|
|