Details of the Drug
General Information of Drug (ID: DMHKS94)
| Drug Name |
Valaciclovir
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Talavir; VACV; ValACV; Valacyclovir; Virval; Zelitrex; Valaciclovir Hcl; Valacyclover Hydrochloric; Valacyclover Hydrochloride; BW256U87; TBB067866; Acyclovir-valine; BW-256U; Valaciclovir (INN); Valaciclovir [INN:BAN]; Valaciclovir, Valtrex; Valtrex (TN); Zelitrex (TN); Valacyclovir, (L)-isomer; L-Valine ester with 9-((2-hydroxyethoxy)methyl)guanine; L-Valine, 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)ethyl ester; L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9 H-purin-9-yl)methoxy]ethyl ester, monohydrochloride; 2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl L-valinate; 2-[(2-amino-6-oxo-3H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate; 2-{[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methyl]oxy}ethyl L-valinate
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Herpes simplex virusHumans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
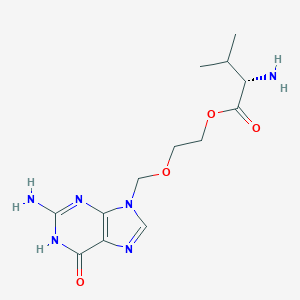 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 324.34 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.9 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Valaciclovir (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Interventions for men and women with their first episode of genital herpes. Cochrane Database Syst Rev. 2016 Aug 30;2016(8):CD010684. | ||||
|---|---|---|---|---|---|
| 2 | Interventions for prevention of herpes simplex labialis (cold sores on the lips). Cochrane Database Syst Rev. 2015 Aug 7;2015(8):CD010095. | ||||
| 3 | Chickenpox. BMJ Clin Evid. 2011 Apr 11;2011:0912. | ||||
| 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4824). | ||||
| 5 | Bayer Inc: VISANNE (2mg dienogest tablets) Product Monograph | ||||
| 6 | BDDCS applied to over 900 drugs | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Extensive oral shedding of human herpesvirus 8 in a renal allograft recipient. Oral Microbiol Immunol. 2009 Apr;24(2):109-15. | ||||
| 9 | Transport of amino acid-based prodrugs by the Na+- and Cl(-) -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004 Mar;308(3):1138-47. | ||||
| 10 | Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998 May 19;246(2):470-5. | ||||
| 11 | Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J Pharmacol Exp Ther. 2002 Mar;300(3):918-24. | ||||
| 12 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 13 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 14 | Bentley ML, Corwin HL, Dasta J "Drug-induced acute kidney injury in the critically ill adult: recognition and prevention strategies." Crit Care Med 38(6 Suppl) (2010): S169-74. [PMID: 20502171] | ||||
| 15 | Laskin OL, de Miranda P, King DH, et al "Effects of probenecid on the pharmacokinetics and elimination of acyclovir in humans." Antimicrob Agents Chemother 21 (1982): 804-7. [PMID: 7103460] | ||||
| 16 | Product Information. Canasa (mesalamine (5-aminosalicylic acid)). Axcan Scandipharm Inc, Birmingham, AL. | ||||
| 17 | Product Information. Clolar (clofarabine). sanofi-aventis, Bridgewater, NJ. | ||||
| 18 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
