| 1 |
ClinicalTrials.gov (NCT01505114) Evaluating the Safety and Tolerability of Antiretroviral Drug Regimens Used as Pre-Exposure Prophylaxis to Prevent HIV Infection in At-Risk Men Who Have Sex With Men and in At-Risk Women
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 806).
|
| 3 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 4 |
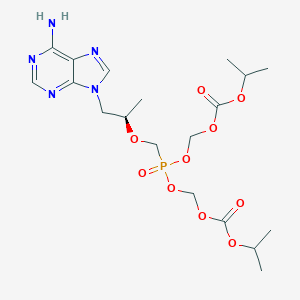
FDA label of Bictegravir, emtricitabine, and tenofovir alafenamide. The 2020 official website of the U.S. Food and Drug Administration.
|
| 5 |
Rapamycin enhances aplaviroc anti-HIV activity: implications for the clinical development of novel CCR5 antagonists. Antiviral Res. 2009 Jul;83(1):86-9.
|
| 6 |
Molecular cloning and radioligand binding characterization of the chemokine receptor CCR5 from rhesus macaque and human. Biochem Pharmacol. 2005 Dec 19;71(1-2):163-72.
|
| 7 |
Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition.
|
| 8 |
ClinicalTrials.gov (NCT04965662) The Role of Home Packs of HIV PEPSE in High Risk Individuals
|
|
|
|
|
|
|