Details of the Drug
General Information of Drug (ID: DMTL94F)
| Drug Name |
Maraviroc
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
376348-65-1; Selzentry; Celsentri; UK-427857; UK-427,857; UK 427857; UNII-MD6P741W8A; MD6P741W8A; CHEMBL256907; MVC; CHEMBL1201187; CHEBI:63608; 4,4-difluoro-N-[(1S)-3-[(1R,5S)-3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[321]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide; Isopropyl, 4,4-difluoro-N-((1S)-3-{(1R,3s,5S)-3-(3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl)-8-azabicyclo(321)octan-8-yl}-1-phenylpropyl)cyclohexanecarboxamide; Maraviroc [USAN]; Celsentri (TN); Celsentri(TM); PRO 140 & Maraviroc; Selzentry (TN); Selzentry(TM); UK-427,857 maraviroc (MVC); Exo-4,4-Difluoro-N-[3-[3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[321]oct-8-yl]-1(S)-phenylpropyl]cyclohexanecarboxamide; PRO 140 (Anti-CCR5 monoclonal antibody) & exo-4,4-Difluoro-N-[3-[3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[321]oct-8-yl]-1(S)-phenylpropyl]cyclohexanecarboxamide; 4,4-Difluoro-N-((1S)-3-(exo-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo(321)oct-8-yl)-1-phenylpropyl)cyclohexanecarboxamide; [3H]maraviroc
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Human Immunodeficiency Virus
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
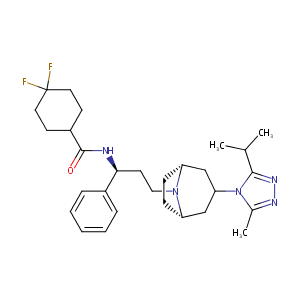 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 513.7 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 12 Disease of the respiratory system | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: CA23 Asthma | |||||||||||||||||||||||||||||||||||||||||
| The Studied Tissue | Lung tissue | |||||||||||||||||||||||||||||||||||||||||
| The Studied Disease | Chronic obstructive pulmonary disease [ICD-11:CA23] | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Maraviroc
Coadministration of a Drug Treating the Disease Different from Maraviroc (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
| DIG |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmaceutical Formulation |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 806). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Rapamycin enhances aplaviroc anti-HIV activity: implications for the clinical development of novel CCR5 antagonists. Antiviral Res. 2009 Jul;83(1):86-9. | ||||
| 6 | Molecular cloning and radioligand binding characterization of the chemokine receptor CCR5 from rhesus macaque and human. Biochem Pharmacol. 2005 Dec 19;71(1-2):163-72. | ||||
| 7 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | ||||
| 8 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 9 | Product Information. Selzentry (maraviroc). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 10 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 11 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 12 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 13 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 14 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 15 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 16 | Canadian Pharmacists Association. | ||||
| 17 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 18 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 19 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 20 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 21 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 22 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 23 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 24 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 25 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
