| 1 |
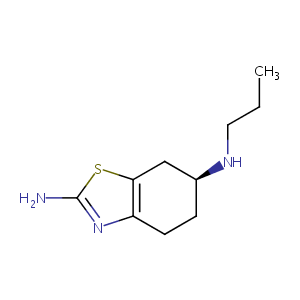
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
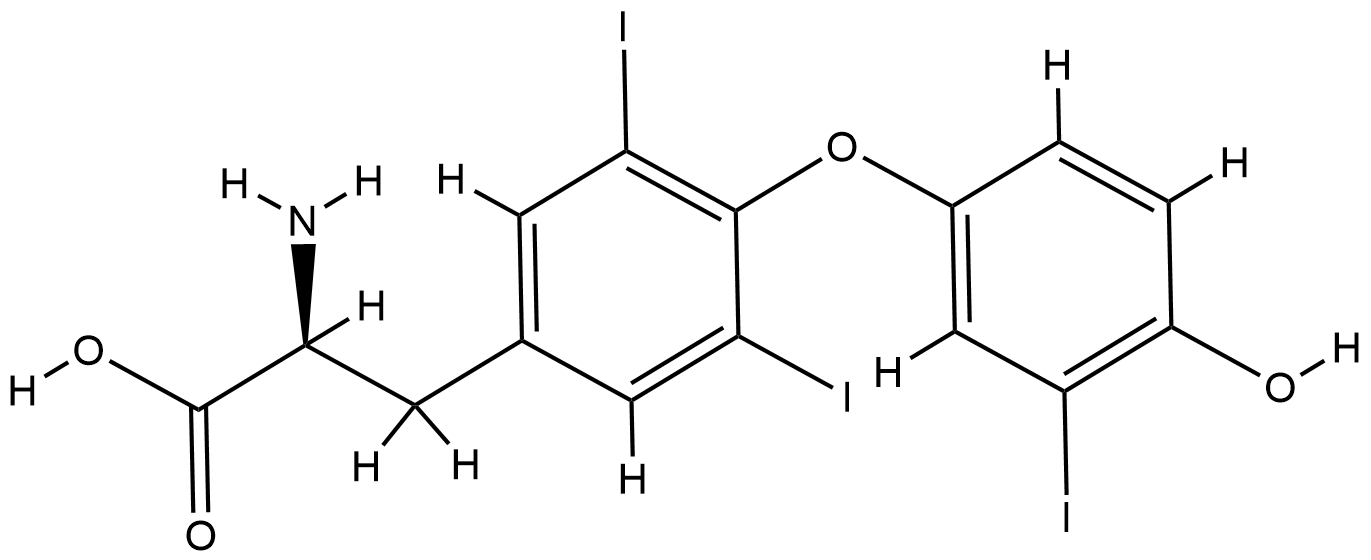
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 953).
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 076923.
|
| 4 |
ClinicalTrials.gov (NCT04348513) Triiodothyronine for the Treatment of Critically Ill Patients With COVID-19 Infection. U.S. National Institutes of Health.
|
| 5 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 6 |
Uptake of pramipexole by human organic cation transporters. Mol Pharm. 2010 Aug 2;7(4):1342-7.
|
| 7 |
OCT1 polymorphism is associated with response and survival time in anti-Parkinsonian drug users. Neurogenetics. 2011 Feb;12(1):79-82.
|
|
|
|
|
|
|