| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
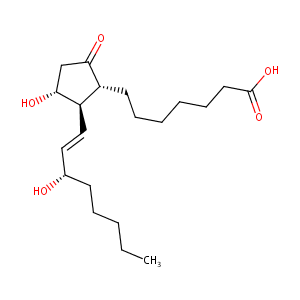
Alprostadil FDA Label
|
| 3 |
Emerging drugs for diabetic foot ulcers. Expert Opin Emerg Drugs. 2006 Nov;11(4):709-24.
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1882).
|
| 5 |
ClinicalTrials.gov (NCT03345095) A Phase III Trial of With Marizomib in Patients With Newly Diagnosed Glioblastoma (MIRAGE). U.S. National Institutes of Health.
|
| 6 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 7 |
Clinical pipeline report, company report or official report of Triphase Accelerator .
|
| 8 |
Arsenic trioxide-mediated antiplatelet activity: pivotal role of the phospholipase C gamma 2-protein kinase C-p38 MAPK cascade. Transl Res. 2010 Feb;155(2):97-108. doi: 10.1016/j.trsl.2009.08.005. Epub 2009 Sep 15.
|
| 9 |
Flow after prostaglandin E1 is mediated by receptor-coupled adenylyl cyclase in human anterior segments. Invest Ophthalmol Vis Sci. 1999 Oct;40(11):2622-6.
|
| 10 |
The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9244-9.
|
| 11 |
Prostaglandin transporter (OATP2A1/SLCO2A1) contributes to local disposition of eicosapentaenoic acid-derived PGE3. Prostaglandins Other Lipid Mediat. 2016 Jan;122:10-7.
|
| 12 |
Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA(hPGT). J Clin Invest. 1996 Sep 1;98(5):1142-9.
|
| 13 |
Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol. 2003 Dec;285(6):F1188-97.
|
| 14 |
Effect of calcium ionophore A23187 on prostaglandin synthase type 2 and 15-hydroxy-prostaglandin dehydrogenase expression in human chorion trophoblast cells. Am J Obstet Gynecol. 2008 Nov;199(5):554.e1-8.
|
| 15 |
Characterization of the recombinant human prostanoid DP receptor and identification of L-644,698, a novel selective DP agonist. Br J Pharmacol. 1998 Apr;123(7):1317-24.
|
| 16 |
Catecholamine and renin-angiotensin response during controlled hypotension induced by prostaglandin E1 combined with hemodilution during isoflurane anesthesia. J Clin Anesth. 1997 Jun;9(4):321-7. doi: 10.1016/s0952-8180(97)00011-1.
|
| 17 |
Effects of prostaglandin E1, dobutamine and placebo on hemodynamic, renal and neurohumoral variables in patients with advanced heart failure. Jpn Heart J. 1999 May;40(3):321-34. doi: 10.1536/jhj.40.321.
|
| 18 |
Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the beta2-adrenergic receptor using phosphoserine-specific antibodies. Mol Pharmacol. 2004 Jan;65(1):196-206. doi: 10.1124/mol.65.1.196.
|
| 19 |
Short-term effects of levosimendan and prostaglandin E1 on hemodynamic parameters and B-type natriuretic peptide levels in patients with decompensated chronic heart failure. Eur J Heart Fail. 2005 Dec;7(7):1156-63. doi: 10.1016/j.ejheart.2005.05.001. Epub 2005 Aug 5.
|
| 20 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 21 |
Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011 Mar;11(3):254-84.
|
|
|
|
|
|
|