| 1 |
ClinicalTrials.gov (NCT04183738) Inflammation and Co-Infections in DEFT
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7365).
|
| 3 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651.
|
| 6 |
Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition.
|
| 7 |
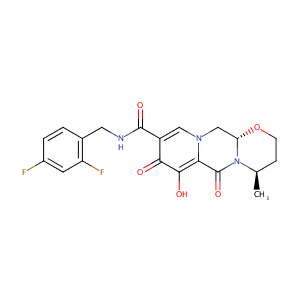
Mechanisms of action, pharmacology and interactions of dolutegravir. Enferm Infecc Microbiol Clin. 2015 Mar;33 Suppl 1:2-8.
|
| 8 |
Dolutegravir Impairs Stem Cell-Based 3D Morphogenesis Models in a Manner Dependent on Dose and Timing of Exposure: An Implication for Its Developmental Toxicity. Toxicol Sci. 2021 Nov 24;184(2):191-203. doi: 10.1093/toxsci/kfab112.
|
| 9 |
2006 drug approvals: finding the niche. Nat Rev Drug Discov. 2007 Feb;6(2):99-101.
|
| 10 |
Impact of drug transporters on cellular resistance towards saquinavir and darunavir. J Antimicrob Chemother. 2010 Nov;65(11):2319-28.
|
| 11 |
Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011 Mar;63(1):157-81.
|
| 12 |
P-glycoprotein mediates efflux transport of darunavir in human intestinal Caco-2 and ABCB1 gene-transfected renal LLC-PK1 cell lines. Biol Pharm Bull. 2009 Sep;32(9):1588-93.
|
| 13 |
Drug interactions with new and investigational antiretrovirals. Clin Pharmacokinet. 2009;48(4):211-41.
|
| 14 |
ClinicalTrials.gov (NCT03017872) Dolutegravir and Darunavir Evaluation in Adults Failing Therapy
|
| 15 |
ClinicalTrials.gov (NCT02486133) Dual Therapy With Boosted Darunavir + Dolutegravir
|
|
|
|
|
|
|