Details of the Drug
General Information of Drug (ID: DMCZGRE)
| Drug Name |
Dolutegravir
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1051375-16-6; GSK1349572; Tivicay; S/GSK1349572; Dolutegravir (GSK1349572); S-349572; GSK-1349572; GSK 1349572; UNII-DKO1W9H7M1; (4r,12as)-N-(2,4-Difluorobenzyl)-7-Hydroxy-4-Methyl-6,8-Dioxo-3,4,6,8,12,12a-Hexahydro-2h-Pyrido[1',2':4,5]pyrazino[2,1-B][1,3]oxazine-9-Carboxamide; CHEBI:76010; DKO1W9H7M1; Tivicay (TN); (4R,12aS)-N-[(2,4-Difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide; Dolutegravir Sodium (
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Human Immunodeficiency VirusHumans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
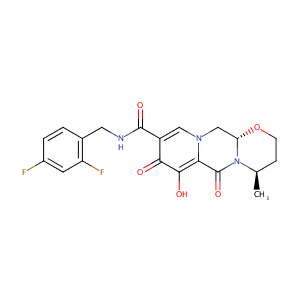 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 419.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Dolutegravir
Coadministration of a Drug Treating the Disease Different from Dolutegravir (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7365). | ||||
|---|---|---|---|---|---|
| 2 | Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2010 Jan;54(1):254-8. doi: 10.1128/AAC.00842-09. Epub 2009 Nov 2. | ||||
| 3 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 4 | Vaishnavi C: Fidaxomicin--the new drug for Clostridium difficile infection. Indian J Med Res. 2015 Apr;141(4):398-407. doi: 10.4103/0971-5916.159251. | ||||
| 5 | Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651. | ||||
| 6 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | ||||
| 7 | Mechanisms of action, pharmacology and interactions of dolutegravir. Enferm Infecc Microbiol Clin. 2015 Mar;33 Suppl 1:2-8. | ||||
| 8 | Dolutegravir Impairs Stem Cell-Based 3D Morphogenesis Models in a Manner Dependent on Dose and Timing of Exposure: An Implication for Its Developmental Toxicity. Toxicol Sci. 2021 Nov 24;184(2):191-203. doi: 10.1093/toxsci/kfab112. | ||||
| 9 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 10 | Cerner Multum, Inc. "Australian Product Information.". | ||||
