| 1 |
ClinicalTrials.gov (NCT00100048) A Study to Evaluate the Safety and Efficacy of an Investigational Drug in HIV Infected Patients (0518-004)(COMPLETED)
|
| 2 |
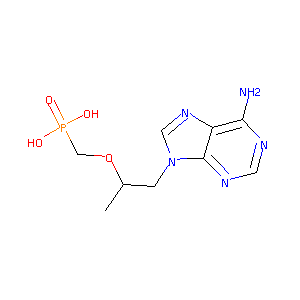
Tenofovir FDA Label
|
| 3 |
Anti-hepatitis B virus activity in vitro of combinations of tenofovir with nucleoside/nucleotide analogues. Antivir Chem Chemother. 2009;19(4):165-76.
|
| 4 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 5 |
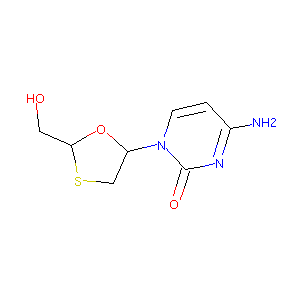
Lamivudine FDA Label
|
| 6 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 7 |
Antiviral drugs in current clinical use. J Clin Virol. 2004 Jun;30(2):115-33.
|
| 8 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665)
|
| 9 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01913)
|
| 10 |
Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007 Feb;71(2):619-27.
|
| 11 |
Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):641-8.
|
| 12 |
Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19(7):687-92.
|
| 13 |
Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis. 2011 Jul 1;204(1):145-53.
|
| 14 |
Tenofovir Disoproxil Fumarate Is a New Substrate of ATP-Binding Cassette Subfamily C Member 11. Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: e01725-16.
|
| 15 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 16 |
The effect of ABCG2 V12M, Q141K and Q126X, known functional variants in vitro, on the disposition of lamivudine. Br J Clin Pharmacol. 2007 Nov;64(5):645-54.
|
| 17 |
Genetic variants of organic cation transporter 1 (OCT1) and OCT2 significantly reduce lamivudine uptake. Biopharm Drug Dispos. 2012 Apr;33(3):170-8.
|
| 18 |
Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos. 2008 Aug;36(8):1616-23.
|
| 19 |
Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res. 2010 Jan;85(1):39-58.
|
| 20 |
Model for intracellular Lamivudine metabolism in peripheral blood mononuclear cells ex vivo and in human immunodeficiency virus type 1-infected adolescents. Antimicrob Agents Chemother. 2006 Aug;50(8):2686-94.
|
| 21 |
Transport of lamivudine [(-)-beta-L-2',3'-dideoxy-3'-thiacytidine] and high-affinity interaction of nucleoside reverse transcriptase inhibitors with human organic cation transporters 1, 2, and 3. J Pharmacol Exp Ther. 2009 Apr;329(1):252-61. doi: 10.1124/jpet.108.146225. Epub 2009 Jan 13.
|
| 22 |
Nucleoside reverse transcriptase inhibitors induce a mitophagy-associated endothelial cytotoxicity that is reversed by coenzyme Q10 cotreatment. Toxicol Sci. 2013 Aug;134(2):323-34. doi: 10.1093/toxsci/kft105. Epub 2013 May 2.
|
| 23 |
Zidovudine induces S-phase arrest and cell cycle gene expression changes in human cells. Mutagenesis. 2005 Mar;20(2):139-46. doi: 10.1093/mutage/gei019. Epub 2005 Mar 22.
|
| 24 |
Effect of lamivudine treatment on plasma levels of transforming growth factor beta1, tissue inhibitor of metalloproteinases-1 and metalloproteinase-1 in patients with chronic hepatitis B. World J Gastroenterol. 2004 Sep 15;10(18):2661-5. doi: 10.3748/wjg.v10.i18.2661.
|
| 25 |
Lamivudine plus interleukin-12 combination therapy in chronic hepatitis B: antiviral and immunological activity. Hepatology. 2005 Nov;42(5):1028-36. doi: 10.1002/hep.20888.
|
| 26 |
Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001 Dec;35(6):749-55. doi: 10.1016/s0168-8278(01)00218-5.
|
| 27 |
Binding of anti-HIV drugs to human serum albumin. IUBMB Life. 2004 Oct;56(10):609-14. doi: 10.1080/15216540400016286.
|
| 28 |
Serotonin transporter mRNA expression is decreased by lamivudine and ribavirin and increased by interferon in immune cells. Scand J Immunol. 2006 Feb;63(2):106-15. doi: 10.1111/j.1365-3083.2005.01715.x.
|
| 29 |
ClinicalTrials.gov (NCT03988452) Nucleosides And Darunavir/Dolutegravir In Africa
|
| 30 |
ClinicalTrials.gov (NCT02369965) Test Albuvirtide in Experienced Patients. U.S. National Institutes of Health.
|
|
|
|
|
|
|