Details of the Drug
General Information of Drug (ID: DM1IS6U)
| Drug Name |
Tenofovir
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Apropovir; PMPA; TFV; Tenefovir; GS 1275; GS 1278; GS1278; GNA & Tenofovir; HHA & Tenofovir; KS-5021; Viread (TN); Viread, Tenofovir; D,L-Tenofovir; PMPA-(R); Phosphonic acid, [[2-(6-amino-9H-purin-9; [(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic acid; Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]-(9CI); Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]-& Galanthus nivalis agglutinin (GNA); Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]-& Hippeastrum hybrid agglutinin(HHA); (R)-9-(2-Phosphonomethoxypropyl)adenine; (R)-9-(2-Phosphonylmethoxypropyl)adenine; (R)-PMPA
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Herpes simplex virusHIV-1Hepatitis B virus
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
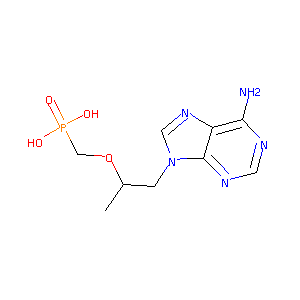 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 287.21 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.6 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
