| 1 |
ClinicalTrials.gov (NCT00112476) Temsirolimus and Bryostatin 1 in Treating Patients With Unresectable or Metastatic Solid Tumors
|
| 2 |
ClinicalTrials.gov (NCT03560245) A Randomized, Double-Blind, Placebo-Controlled, Phase 2 Study Assessing the Safety, Tolerability and Efficacy of Bryostatin in the Treatment of Moderately Severe to Severe Alzheimer's Disease Subjects Not Receiving Memantine Treatment. U.S.National Institutes of Health.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5892).
|
| 4 |
Protein kinase epsilon dampens the secretory response of model intestinal epithelia during ischemia. Surgery. 2001 Aug;130(2):310-8.
|
| 5 |
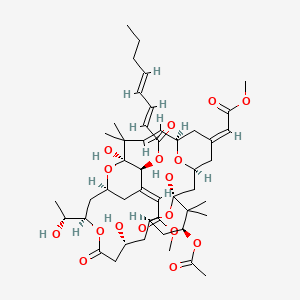
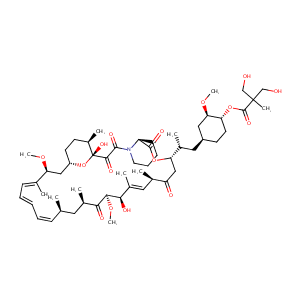
Bryostatin-1: a promising compound for neurological disorders. Front Pharmacol. 2023 Jun 7;14:1187411.
|
| 6 |
Modulation of protein kinase C activity and calcium-sensitive isoform expression in human myeloid leukemia cells by bryostatin 1: relationship to differentiation and ara-C-induced apoptosis. Exp Cell Res. 1996 Oct 10;228(1):65-75. doi: 10.1006/excr.1996.0300.
|
| 7 |
Elucidating mechanisms of toxicity using phenotypic data from primary human cell systems--a chemical biology approach for thrombosis-related side effects. Int J Mol Sci. 2015 Jan 5;16(1):1008-29. doi: 10.3390/ijms16011008.
|
| 8 |
Phase II trial of bryostatin 1 in patients with relapsed low-grade non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Clin Cancer Res. 2000 Mar;6(3):825-8.
|
| 9 |
Effect of serum and antioxidants on the immunogenicity of protein kinase C-activated chronic lymphocytic leukemia cells. J Immunother. 2005 Jan-Feb;28(1):28-39. doi: 10.1097/00002371-200501000-00004.
|
| 10 |
Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J Biol Chem. 2003 Dec 19;278(51):51091-9. doi: 10.1074/jbc.M306541200. Epub 2003 Oct 3.
|
| 11 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 12 |
Advances in kinase targeting: current clinical use and clinical trials. Trends Pharmacol Sci. 2014 Nov;35(11):604-20.
|
| 13 |
Association of NR1I2, CYP3A5 and ABCB1 genetic polymorphisms with variability of temsirolimus pharmacokinetics and toxicity in patients with metastatic bladder cancer. Cancer Chemother Pharmacol. 2017 Sep;80(3):653-659.
|
| 14 |
Drug Interactions Flockhart Table
|
|
|
|
|
|
|