Details of the Drug
General Information of Drug (ID: DM1JOXY)
| Drug Name |
Bryostatin-1
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bryostatin 1; 83314-01-6; Bryostatin-1; NSC 339555; CHEMBL449158; CHEBI:88353; 37O2X55Y9E; BRYOSTATIN; NSC-339555; 2,4-Octadienoic acid, (1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,21R,23R,25S)-25-(acetyloxy)-1,11,21-trihydroxy-17-((1R)-1-hydroxyethyl)-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo(21.3.1.13,7.111,15)nonacos-8-en-12-yl ester, (2E,4E)-; DTXSID8046876; UNII-37O2X55Y9E; BRN 4349157; BRYOSTATIN 1 [MI]; SCHEMBL182960; BRYOSTATIN 1 [WHO-DD]; Bryostatin 1, >=99%, solid; MJQUEDHRCUIRLF-TVIXENOKSA-N; BDBM50258529; BMY-45618; MFCD00893832; Bryostatin 1 - CAS 83314-01-6; (1S-(1R*,3R*,5Z,7S*,8E,11R*,12R*(2E,4E),13E,15R*,17S*(S*),21S*,23S*,25R*))-25-(Acetyloxy)-1,11,21-trihydroxy-17-(1-hydroxyethyl)-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo(21.3.1.13,7.111,15)nonacos-8-en-12-yl 2,4-octadienoate; 2,4-Octadienoic acid, 25-(acetyloxy)-1,11,21-trihydroxy-17-(1-hydroxyethyl)-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo(21.3.1.13,7.111,15)nonacos-8-en-12-yl ester, (1S-(1R*,3R*,5Z,7S*,8E,11R*,12R*(2E,4E),13E,15R*,17S*(S*),21S*,23S*,25R*))-; HY-105231; CS-0025440; Q27095907; (1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,21R,23R,25S)-25-(acetyloxy)-1,11,21-trihydroxy-17-[(1R)-1-hydroxyethyl]-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo[21.3.1.1(3,7).1(11,15)]nonacos-8-en-12-yl (2E,4E)-octa-2,4-dienoate; (1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,21R,23R,25S)-25-(Acetyloxy)-1,11,21-trihydroxy-17-[(1R)-1-hydroxyethyl]-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo[21.3.1.13,7.111,15]nonacos-8-en-12-yl-(2E, 4E)-2,4-octadienoic acid ester; [(1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,21R,23R,25S)-25-acetyloxy-1,11,21-trihydroxy-17-[(1R)-1-hydroxyethyl]-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo[21.3.1.13,7.111,15]nonacos-8-en-12-yl] (2E,4E)-octa-2,4-dienoate
|
|||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||
| Drug Type |
Small molecule
|
|||||||||||||||||||||||||
| Structure |
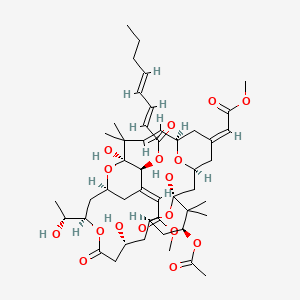 |
|||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | |||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Alzheimer disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
References
| 1 | ClinicalTrials.gov (NCT03560245) A Randomized, Double-Blind, Placebo-Controlled, Phase 2 Study Assessing the Safety, Tolerability and Efficacy of Bryostatin in the Treatment of Moderately Severe to Severe Alzheimer's Disease Subjects Not Receiving Memantine Treatment. U.S.National Institutes of Health. | ||||
|---|---|---|---|---|---|
| 2 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 3 | Bryostatin-1: a promising compound for neurological disorders. Front Pharmacol. 2023 Jun 7;14:1187411. | ||||
| 4 | Protein kinase epsilon dampens the secretory response of model intestinal epithelia during ischemia. Surgery. 2001 Aug;130(2):310-8. | ||||
| 5 | Phase II trial of bryostatin 1 in patients with relapsed low-grade non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Clin Cancer Res. 2000 Mar;6(3):825-8. | ||||
| 6 | Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J Biol Chem. 2003 Dec 19;278(51):51091-9. doi: 10.1074/jbc.M306541200. Epub 2003 Oct 3. | ||||
| 7 | Effect of serum and antioxidants on the immunogenicity of protein kinase C-activated chronic lymphocytic leukemia cells. J Immunother. 2005 Jan-Feb;28(1):28-39. doi: 10.1097/00002371-200501000-00004. | ||||
| 8 | Modulation of protein kinase C activity and calcium-sensitive isoform expression in human myeloid leukemia cells by bryostatin 1: relationship to differentiation and ara-C-induced apoptosis. Exp Cell Res. 1996 Oct 10;228(1):65-75. doi: 10.1006/excr.1996.0300. | ||||
| 9 | Elucidating mechanisms of toxicity using phenotypic data from primary human cell systems--a chemical biology approach for thrombosis-related side effects. Int J Mol Sci. 2015 Jan 5;16(1):1008-29. doi: 10.3390/ijms16011008. | ||||
