| 1 |
ClinicalTrials.gov (NCT02774837) Tenofovir Versus Tenofovir + Telbivudine for Chronic Hepatitis B
|
| 2 |
Nucleoside and nucleotide analogs in the treatment of chronic hepatitis B. Enferm Infecc Microbiol Clin. 2008 May;26 Suppl 7:32-8.
|
| 3 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 4 |
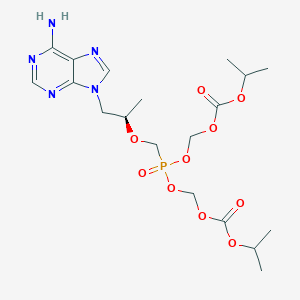
FDA label of Bictegravir, emtricitabine, and tenofovir alafenamide. The 2020 official website of the U.S. Food and Drug Administration.
|
| 5 |
Nucleos(t)ide analogues for hepatitis B virus: strategies for long-term success. Best Pract Res Clin Gastroenterol. 2008;22(6):1081-92.
|
| 6 |
Insights into the mechanisms of telbivudine-induced myopathy associated with mitochondrial dysfunction. Chem Biol Interact. 2023 Sep 25;383:110692. doi: 10.1016/j.cbi.2023.110692. Epub 2023 Sep 1.
|
| 7 |
ClinicalTrials.gov (NCT03468907) The Safety of Anti-viral Therapy in Preventing HBV MTCT in Pregnant Women After Discontinuation
|
|
|
|
|
|
|