| 1 |
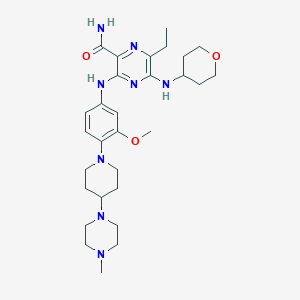
ClinicalTrials.gov (NCT02014558) Dose Escalation Study Investigating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics of ASP2215 in Patients With Relapsed or Refractory Acute Myeloid Leukemia
|
| 2 |
Cephalexin FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4832).
|
| 4 |
2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89.
|
| 5 |
Relationship between penicillin-binding protein patterns and beta-lactamases in clinical isolates of Bacteroides fragilis with different susceptibility to beta-lactam antibiotics. J Med Microbiol. 2004 Mar;53(Pt 3):213-21.
|
| 6 |
Reduced renal clearance of a zwitterionic substrate cephalexin in MATE1-deficient mice. J Pharmacol Exp Ther. 2010 Aug;334(2):651-6.
|
| 7 |
Direct evidence for efficient transport and minimal metabolism of L-cephalexin by oligopeptide transporter 1 in budded baculovirus fraction. Biol Pharm Bull. 2009 Aug;32(8):1459-61.
|
| 8 |
Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. J Pharmacol Exp Ther. 2006 Jul;318(1):455-60.
|
| 9 |
Transport and metabolic characterization of Caco-2 cells expressing CYP3A4 and CYP3A4 plus oxidoreductase. Pharm Res. 1999 Sep;16(9):1352-9.
|
| 10 |
Population pharmacokinetic analysis of mirtazapine. Eur J Clin Pharmacol. 2004 Sep;60(7):473-80.
|
| 11 |
A novel quorum-quenching N-acylhomoserine lactone acylase from Acidovorax sp. strain MR-S7 mediates antibiotic resistance. Appl Environ Microbiol. 2017 Jun 16;83(13). pii: e00080-17.
|
| 12 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 13 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 14 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665)
|
| 15 |
ClinicalTrials.gov (NCT02456883) Study to Investigate the Absorption, Metabolism and Excretion of [14C] ASP2215 in Patients With Advanced Solid Tumors.
|
|
|
|
|
|
|