| 1 |
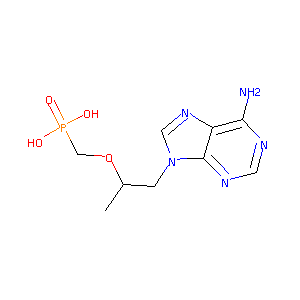
ClinicalTrials.gov (NCT01639066) Tenofovir Plus Entecavir vs. Tenofovir in Adefovir-Resistant Chronic Hepatitis B
|
| 2 |
Tenofovir FDA Label
|
| 3 |
Anti-hepatitis B virus activity in vitro of combinations of tenofovir with nucleoside/nucleotide analogues. Antivir Chem Chemother. 2009;19(4):165-76.
|
| 4 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 5 |
Antiviral drugs in current clinical use. J Clin Virol. 2004 Jun;30(2):115-33.
|
| 6 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665)
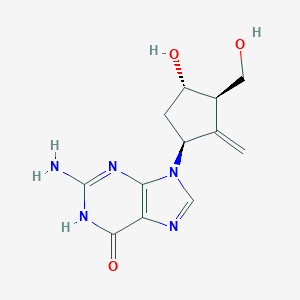
|
| 7 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01913)
|
| 8 |
Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007 Feb;71(2):619-27.
|
| 9 |
Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):641-8.
|
| 10 |
Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19(7):687-92.
|
| 11 |
Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis. 2011 Jul 1;204(1):145-53.
|
| 12 |
Tenofovir Disoproxil Fumarate Is a New Substrate of ATP-Binding Cassette Subfamily C Member 11. Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: e01725-16.
|
| 13 |
Effect of hepatitis B virus reverse transcriptase variations on entecavir treatment response. J Infect Dis. 2014 Sep 1;210(5):701-7.
|
| 14 |
Multiple SLC and ABC Transporters Contribute to the Placental Transfer of Entecavir. Drug Metab Dispos. 2017 Mar;45(3):269-278.
|
| 15 |
Human organic anion transporter 2 is an entecavir, but not tenofovir, transporter. Drug Metab Pharmacokinet. 2017 Feb;32(1):116-119.
|
| 16 |
The oligopeptide transporter 2-mediated reabsorption of entecavir in rat kidney. Eur J Pharm Sci. 2014 Feb 14;52:41-7.
|
| 17 |
ClinicalTrials.gov (NCT00410202) Entecavir Plus Adefovir Combination Therapy Versus Entecavir Monotherapy vs Therapy With Adefovir Plus Lamivudine for Chronic Hepatitis B Infected Subjects With Lamivudine-resistant Virus
|
|
|
|
|
|
|