Details of the Drug Combinations
General Information of This Drug (ID: DM7ICNU)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Atridox; Azudoxat; DOXY; Deoxymykoin; Dossiciclina; Doxiciclina; Doxitard; Doxivetin; Doxycen; Doxychel; Doxycin; Doxycyclin; Doxycyclinum; Doxysol; Doxytec; Doxytetracycline; Hydramycin; Investin; Jenacyclin; Liviatin; Monodox; Oracea; Ronaxan; Spanor; Supracyclin; Vibramycin; Vibramycine; Vibravenos; DOXCYCLINE ANHYDROUS; DOXYCYCLINE CALCIUM; DOXYCYCLINE MONOHYDRATE; Dossiciclina [DCIT]; Doxiciclina [Italian]; Doxycycline anhydrous; Doxycycline hyclate; Vibramycin Novum; Alpha-Doxycycline; Alti-Doxycycline; Apo-Doxy; BMY-28689; BU-3839T; Doxiciclina [INN-Spanish]; Doxy-Caps; Doxy-Puren; Doxy-Tabs; Doxychel (TN); Doxycycline (INN); Doxycycline (TN); Doxycycline (anhydrous); Doxycycline (internal use); Doxycycline-Chinoin; Doxycyclinum [INN-Latin]; Novo-Doxylin; Nu-Doxycycline; Periostat (TN); Vibra-tabs; Alpha-6-Deoxyoxytetracycline; DMSC (*Fosfatex); Doxycycline (200mg/day) or Placebo; Monodox (*monohydrate); Vibramycin (*monohydrate); Vivox (*Hyclate); GS-3065 (*monohydrate); Alpha-6-Deoxy-5-hydroxytetracycline; (2E,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2Z)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2Z,4S,4aR,5S,5aR,6R)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2Z,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (4S,4aR,5S,5aR,6R,12aS)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide; 2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4S,4aR,5S,5aR,6R,12aS); 5-Hydroxy-alpha-6-deoxytetracycline; 6-Deoxyoxytetracycline; 6-Deoxytetracycline; 6-alpha-Deoxy-5-oxytetracycline; 6alpha-Deoxy-5-oxytetracycline
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
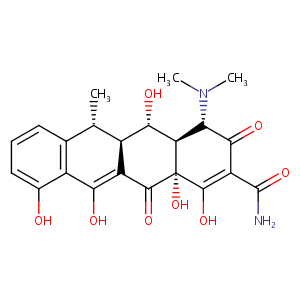 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
10 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
33 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
