| 1 |
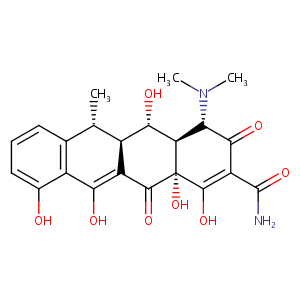
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Doxycycline FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6464).
|
| 4 |
ClinicalTrials.gov (NCT00764361) Safety Study of Topical Doxycycline Gel for Adult Diabetic Lower Extremity Ulcers. U.S. National Institutes of Health.
|
| 5 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 019578
|
| 6 |
ClinicalTrials.gov (NCT04347031) An Open Randomized Study of the Effectiveness of Mefloquine for the Treatment of Patients With COVID19. U.S. National Institutes of Health.
|
| 7 |
Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014 Aug;58(8):4885-93.
|
| 8 |
Mefloquine FDA Label
|
| 9 |
Aryl hydrocarbon receptor protects lung adenocarcinoma cells against cigarette sidestream smoke particulates-induced oxidative stress. Toxicol Appl Pharmacol. 2012 Mar 15;259(3):293-301.
|
| 10 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 11 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 12 |
A further interaction study of quinine with clinically important drugs by human liver microsomes: determinations of inhibition constant (Ki) and type of inhibition. Eur J Drug Metab Pharmacokinet. 1999 Jul-Sep;24(3):272-8.
|
| 13 |
Functional and histochemical analysis of MDR3 P-glycoprotein in a tetracycline-controlled gene expression system. Eur J Med Res. 2000 Dec 29;5(12):517-22.
|
| 14 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 15 |
Synthesis and in vitro evaluation of targeted tetracycline derivatives: effects on inhibition of matrix metalloproteinases. Bioorg Med Chem. 2007 Mar 15;15(6):2368-74. doi: 10.1016/j.bmc.2007.01.026. Epub 2007 Jan 19.
|
| 16 |
Chloramphenicol causes mitochondrial stress, decreases ATP biosynthesis, induces matrix metalloproteinase-13 expression, and solid-tumor cell invasion. Toxicol Sci. 2010 Jul;116(1):140-50. doi: 10.1093/toxsci/kfq085. Epub 2010 Mar 25.
|
| 17 |
A high-content screen identifies novel compounds that inhibit stress-induced TDP-43 cellular aggregation and associated cytotoxicity. J Biomol Screen. 2014 Jan;19(1):44-56. doi: 10.1177/1087057113501553. Epub 2013 Sep 9.
|
| 18 |
An ultrastructural study of the effects of mefloquine on malaria parasites. Am J Trop Med Hyg. 1987 Jan;36(1):9-14.
|
| 19 |
Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004 Jan;75(1):13-33.
|
| 20 |
Effect of rifampin on plasma concentrations of mefloquine in healthy volunteers. J Pharm Pharmacol. 2000 Oct;52(10):1265-9.
|
|
|
|
|
|
|