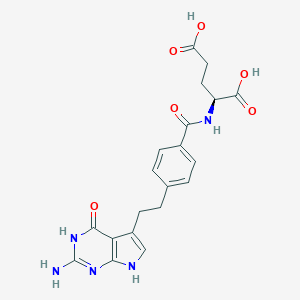
| 1 |
Pemetrexed FDA Label
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6837).
|
| 3 |
ClinicalTrials.gov (NCT03566576) Phase I Study of the Combination of Anlotinib With Pemetrexed or Docetaxel
|
| 4 |
ClinicalTrials.gov (NCT02535312) Methoxyamine, Cisplatin, and Pemetrexed Disodium in Treating Patients With Advanced Solid Tumors or Mesothelioma That Cannot Be Removed by Surgery or Mesothelioma That Is Refractory to Pemetrexed Disodium and Cisplatin or Carboplatin
|
| 5 |
ClinicalTrials.gov (NCT01397305) An Extended Feasibility Phase I/II Study of Methylenetetrahydrofolate (Arfolitixorin) and Pemetrexed Single Agent, Given as Neoadjuvant Treatment in Patients With Resectable Rectal Cancer
|
| 6 |
ClinicalTrials.gov (NCT04173338) Cabozantinib With Pemetrexed in Advanced Non-small Cell Lung Cancer, Urothelial Cancer and Malignant Mesothelioma
|
| 7 |
ClinicalTrials.gov (NCT00230542) Carboplatin and Pemetrexed in Recurrent Platinum Sensitive Ovarian Cancer
|
| 8 |
ClinicalTrials.gov (NCT00101283) Pemetrexed Plus Gemcitabine or Carboplatin for Patients With Advanced Malignant Pleural Mesothelioma
|
| 9 |
ClinicalTrials.gov (NCT02039674) A Study of Pembrolizumab (MK-3475) in Combination With Chemotherapy or Immunotherapy in Participants With Non-small Cell Lung Cancer (MK-3475-021/KEYNOTE-021)
|
| 10 |
ClinicalTrials.gov (NCT00609518) A Study for Patients With Non-Squamous Non-Small Cell Lung Cancer
|
| 11 |
ClinicalTrials.gov (NCT01172028) Pemetrexed Disodium and Docetaxel in Treating Patients With Advanced Solid Tumors
|
| 12 |
ClinicalTrials.gov (NCT00660816) Pemetrexed or Docetaxel With or Without Erlotinib in Stage IIIB or Stage IV Non-Small Cell Lung Cancer
|
| 13 |
ClinicalTrials.gov (NCT00684099) Docetaxel/Pemetrexed as 1st Line Treatment in Patients With Non Small Cell Lung Cancer (NSCLC)
|
| 14 |
ClinicalTrials.gov (NCT00530621) Study of Enzastaurin Versus Placebo With Pemetrexed for Participants With Advanced or Metastatic Lung Cancer
|
| 15 |
ClinicalTrials.gov (NCT00447057) Study of Pemetrexed Versus Pemetrexed Plus Erlotinib as Treatment of Nonsquamous Non-Small Cell Lung Cancer (NSCLC)
|
| 16 |
ClinicalTrials.gov (NCT00387322) Pemetrexed Disodium and Erlotinib in Treating Patients With Advanced Non-Small Cell Lung Cancer or Other Solid Tumors
|
| 17 |
ClinicalTrials.gov (NCT00550173) A Study for Non-Smoker Patients With Nonsquamous Non-Small Cell Lung Cancer
|
| 18 |
ClinicalTrials.gov (NCT00434174) Safety of Everolimus and Pemetrexed in Lung Cancer Patients
|
| 19 |
ClinicalTrials.gov (NCT01469000) A Study of Pemetrexed and Gefitinib Versus Gefitinib in Non-Small Cell Lung Cancer (NSCLC)
|
| 20 |
ClinicalTrials.gov (NCT00063570) Pemetrexed Plus Gemcitabine in Metastatic Breast Cancer Patients After Receiving Taxane Therapy
|
| 21 |
ClinicalTrials.gov (NCT00193414) Pemetrexed and Gemcitabine in Patients With Advanced Non-Small Cell Lung Cancer
|
| 22 |
ClinicalTrials.gov (NCT00769600) Phase II Study of Itraconazole and Pemetrexed in Patients With Previously Treated Non-Squamous NSCLC
|
| 23 |
ClinicalTrials.gov (NCT01170871) Ixabepilone and Pemetrexed/Solid Tumors
|
| 24 |
ClinicalTrials.gov (NCT00528281) A Phase II Trial of Lapatinib (TYKERB) + Pemetrexed (ALIMTA) in Advanced Non Small Cell Lung Cancer With an Initial Dose Finding Phase
|
| 25 |
ClinicalTrials.gov (NCT00988858) A Study of Advanced or Metastatic Non-small Cell Lung Cancer
|
| 26 |
ClinicalTrials.gov (NCT02124148) A Study of Prexasertib (LY2606368) With Chemotherapy or Targeted Agents in Participants With Advanced Cancer
|
| 27 |
ClinicalTrials.gov (NCT02079636) A Study of Abemaciclib (LY2835219) in Combination With Another Anti-cancer Drug in Participants With Lung Cancer (NSCLC)
|
| 28 |
ClinicalTrials.gov (NCT00907179) Pemetrexed and LBH589 in Patients With Advanced Non-Small Cell Lung Cancer
|
| 29 |
ClinicalTrials.gov (NCT00619424) A Phase I Study Of Pazopanib With Either Erlotinib Or Pemetrexed In Patients With Advanced Solid Tumors
|
| 30 |
ClinicalTrials.gov (NCT00390000) Vatalanib and Pemetrexed Disodium in Treating Patients With Advanced Solid Tumors
|
| 31 |
ClinicalTrials.gov (NCT00921310) Temsirolimus and Pemetrexed for Recurrent or Refractory Non-Small Cell Lung Cancer
|
| 32 |
ClinicalTrials.gov (NCT00612677) Pemetrexed &Oxaliplatin in Patients w Recurrent NSCLCa After Failure to Platinum Based Adjuvant Chem
|
| 33 |
ClinicalTrials.gov (NCT00418886) Efficacy Study Comparing ZD6474 in Combination With Pemetrexed and Pemetrexed Alone in 2nd Line NSCLC Patients
|
| 34 |
ClinicalTrials.gov (NCT00191984) A Study of the Combination of Pemetrexed and Irinotecan Every Two Weeks in Metastatic Colorectal Cancer
|
| 35 |
ClinicalTrials.gov (NCT00470405) Pemetrexed and Oxaliplatin in Treating Patients With Metastatic Solid Tumors or Lymphoma
|
| 36 |
ClinicalTrials.gov (NCT00320073) Vinflunine and Erlotinib or Pemetrexed in Treating Patients With Unresectable or Metastatic Solid Tumors
|
| 37 |
ClinicalTrials.gov (NCT00506051) ZD6474(Vandetanib) + Alimta Combo Study
|
| 38 |
ClinicalTrials.gov (NCT03023319) Bosutinib in Combination With Pemetrexed in Patients With Selected Metastatic Solid Tumors
|
| 39 |
ClinicalTrials.gov (NCT00408226) Safety Study of MKC-1 Combined With Pemetrexed to Treat Advanced Cancer and Non-Small Cell Lung Cancer
|
| 40 |
ClinicalTrials.gov (NCT01783197) Study of Selumetinib in Patients With Previously Treated or Untreated Advanced/Metastatic NSCLC
|
| 41 |
ClinicalTrials.gov (NCT01215916) A Phase 1 Study in Patients With Solid Tumors
|
| 42 |
ClinicalTrials.gov (NCT01985451) Pemetrexed and Temozolomide in Treating Patients With Relapsed Primary Central Nervous System Lymphoma PCNSL?Unknown status
|
| 43 |
ClinicalTrials.gov (NCT01192165) Safety and Tolerability Study of GSK1120212, a MEK Inhibitor, in Combination With Docetaxel, Erlotinib, Pemetrexed, Pemetrexed + Carboplatin, Pemetrexed + Cisplatin, or Nab-Paclitaxel
|
| 44 |
ClinicalTrials.gov (NCT00745875) ZD4054 (Zibotentan) Phase II Non-Small Cell Lung Cancer Study
|
| 45 |
ClinicalTrials.gov (NCT02296671) Personalized Therapy for Esophagogastric Cancer Using Thymidylate Synthase Genetic Markers
|
| 46 |
ClinicalTrials.gov (NCT03190239) Efficacy and Safety of Maintenance Apatinib Combined With Pemetrexed in Advanced Non-squamous Non-small Cell Lung Cancer Patients
|
| 47 |
ClinicalTrials.gov (NCT05681195) Zanubrutinib With Pemetrexed to Treat Relapsed/Refractory Primary and Secondary Central Nervous System (CNS) Lymphomas
|
| 48 |
ClinicalTrials.gov (NCT02518802) Pemetrexed Combined With Synchronous Gefitinib as Adjuvent Therapy in Patient With EGFR Mutant Lung Adenocarcinoma
|
|
|
|
|
|
|