| 1 |
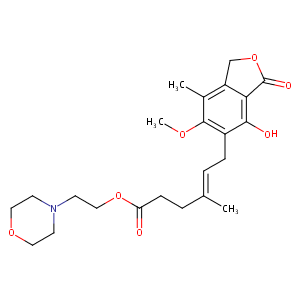
Mycophenolate mofetil FDA Label
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6831).
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 341).
|
| 4 |
High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses. J Virol. 2019 May 29;93(12). pii: e00023-19.
|
| 5 |
ClinicalTrials.gov (NCT01041508) Clofarabine and Low Dose Total Body Irradiation as a Preparative Regimen for Stem Cell Transplant in Leukemia.
|
| 6 |
ClinicalTrials.gov (NCT01962636) Umbilical Cord Blood Transplantation Using a Myeloablative Preparative Regimen for Hematological Diseases
|
| 7 |
ClinicalTrials.gov (NCT01047072) Low-Dose Conditioning Followed by Donor Stem Cell Transplant in Treating Patients With Severe Systemic Sclerosis
|
| 8 |
ClinicalTrials.gov (NCT02548468) Reduced Intensity Conditioning Before Partially Matched Donor Stem Cell Transplant in Treating Patients With Advanced Cutaneous T Cell Lymphoma
|
| 9 |
ClinicalTrials.gov (NCT01342289) Shorter Course Tacrolimus After Nonmyeloablative, Related Donor BMT With High-dose Posttransplantation Cyclophosphamide
|
| 10 |
ClinicalTrials.gov (NCT00686556) Total Marrow Irradiation for Refractory Acute Leukemia
|
| 11 |
ClinicalTrials.gov (NCT00376519) Umbilical Cord Blood T-Regulatory Cell Infusion Followed by Donor Umbilical Cord Blood Transplant in Treating Patients With High-Risk Leukemia or Other Hematologic Diseases
|
| 12 |
ClinicalTrials.gov (NCT00343798) A Pilot Study to Evaluate the Co-Infusion of Ex Vivo Expanded Cord Blood Cells With an Unmanipulated Cord Blood Unit in Patients Undergoing Cord Blood Transplant for Hematologic Malignancies
|
| 13 |
ClinicalTrials.gov (NCT00429143) A Two-Step Approach to Bone Marrow Transplant Using Cells From A Partially-Matched Relative
|
| 14 |
ClinicalTrials.gov (NCT00354419) Cyclophosphamide, Antithymocyte Globulin, and Total-Body Irradiation in Treating Patients With Severe Aplastic Anemia Undergoing Umbilical Cord Blood Transplant
|
| 15 |
ClinicalTrials.gov (NCT02487069) Allo-HSCT With Alternative Donor in Treatment of Hematologic Malignancy
|
| 16 |
ClinicalTrials.gov (NCT03791827) Multicenter Registry of Pediatric Lupus Nephritis in China
|
| 17 |
ClinicalTrials.gov (NCT00005799) Fludarabine Phosphate, Low-Dose Total Body Irradiation, and Donor Stem Cell Transplant in Treating Patients With Hematologic Malignancies or Kidney Cancer
|
| 18 |
ClinicalTrials.gov (NCT00014235) Fludarabine Phosphate and Total-Body Radiation Followed by Donor Peripheral Blood Stem Cell Transplant and Immunosuppression in Treating Patients With Hematologic Malignancies
|
| 19 |
ClinicalTrials.gov (NCT00359658) Withdrawal of Steroids, Cyclosporine A Dose Reduction and Switch to Mycophenolatmofetile After Heart Transplantation
|
| 20 |
ClinicalTrials.gov (NCT00420537) Shift to Everolimus (RAD) Kidney Sparing Study
|
| 21 |
ClinicalTrials.gov (NCT00117689) Evaluation of Thymoglobulin Induction and Reduced Doses of Calcineurin Inhibitors on Liver Transplant Rejection
|
| 22 |
ClinicalTrials.gov (NCT00089011) Tacrolimus and Mycophenolate Mofetil in Preventing Graft-Versus-Host Disease in Patients Who Have Undergone Total-Body Irradiation With or Without Fludarabine Phosphate Followed by Donor Peripheral Blood Stem Cell Transplant for Hematologic Cancer
|
| 23 |
ClinicalTrials.gov (NCT00425438) A Study of CellCept (Mycophenolate Mofetil) in Patients With Lupus Nephritis.
|
| 24 |
ClinicalTrials.gov (NCT00008450) Total-Body Irradiation Followed By Cyclosporine and Mycophenolate Mofetil in Treating Patients With Severe Combined Immunodeficiency Undergoing Donor Bone Marrow Transplant
|
| 25 |
ClinicalTrials.gov (NCT01801280) Influence of Pantoprazole on the Bioavailability of MMF and EC-MPS
|
| 26 |
ClinicalTrials.gov (NCT00054353) Reduced-Intensity Conditioning Followed By Donor Peripheral Blood Stem Cell Transplant in Treating Patients With Multiple Myeloma
|
| 27 |
ClinicalTrials.gov (NCT00008177) Radiolabeled Monoclonal Antibody Therapy, Fludarabine Phosphate, and Low-Dose Total-Body Irradiation Followed by Donor Stem Cell Transplant and Immunosuppression Therapy in Treating Older Patients With Advanced Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndromes
|
| 28 |
ClinicalTrials.gov (NCT02711826) Treg Therapy in Subclinical Inflammation in Kidney Transplantation
|
| 29 |
ClinicalTrials.gov (NCT00003954) Melphalan and Stem Cell Transplant Before Total-Body Irradiation and Donor Stem Cell Transplant in Treating Patients With Stage I-III Multiple Myeloma
|
| 30 |
ClinicalTrials.gov (NCT05049863) Chemotherapy in Patients With Relapsed Small Cell Lung Cancer in Combination With Allopurinol and MycoPhenolate (CLAMP Trial)
|
| 31 |
ClinicalTrials.gov (NCT00005851) Low-Dose Total-Body Irradiation and Fludarabine Phosphate Followed By Donor Peripheral Blood Stem Cell Transplant in Treating Patients With Stage IV Kidney Cancer
|
| 32 |
ClinicalTrials.gov (NCT02728700) Sirolimus and Mycophenolate Mofetil in Preventing GVHD in Patients With Hematologic Malignancies Undergoing HSCT
|
| 33 |
ClinicalTrials.gov (NCT04477200) Mycophenolate Mofetil Combined With Radiation Therapy in Glioblastoma
|
| 34 |
ClinicalTrials.gov (NCT01252667) Clofarabine and Low-Dose Total-Body Irradiation in Treating Patients With Acute Myeloid Leukemia Undergoing Donor Peripheral Blood Stem Cell Transplant
|
| 35 |
ClinicalTrials.gov (NCT02566304) Reduced Intensity Chemotherapy and Radiation Therapy Before Donor Stem Cell Transplant in Treating Patients With Hematologic Malignancies
|
| 36 |
ClinicalTrials.gov (NCT00049504) Haploidentical Donor Bone Marrow Transplant in Treating Patients With High-Risk Hematologic Cancer
|
| 37 |
ClinicalTrials.gov (NCT01175785) Infusion of Off-the-Shelf Expanded Cord Blood Cells to Augment Cord Blood Transplant in Patients With Hematologic Malignancies
|
| 38 |
ClinicalTrials.gov (NCT02556931) Shorter Course Tacro After NMA, Related Donor PBSCT With High-dose Posttransplant Cy for Hard-to-Engraft Malignancies
|
| 39 |
ClinicalTrials.gov (NCT00309842) Myeloablative Umbilical Cord Blood Transplantation in Hematological Diseases
|
| 40 |
ClinicalTrials.gov (NCT02208037) Novel Approaches for Graft-versus-Host Disease Prevention Compared to Contemporary Controls (BMT CTN 1203)
|
| 41 |
ClinicalTrials.gov (NCT01341301) Donor Stem Cell Transplant in Treating Patients With High-Risk Hematologic Malignancies
|
| 42 |
ClinicalTrials.gov (NCT01349101) A Research Study of Bone Marrow Transplantation From Unrelated or Partially Matched Related Donors
|
| 43 |
ClinicalTrials.gov (NCT03032783) A Two Step Approach to Allogeneic Hematopoietic Stem Cell Transplantation for Patients With Hematologic Malignancies-Increasing GVT Effects Without Increasing Toxicity
|
| 44 |
ClinicalTrials.gov (NCT01982682) Allogeneic Hematopoietic Stem Cell Transplantation for High-Risk Hematologic Malignancies Using One Haploidentical Donor
|
| 45 |
ClinicalTrials.gov (NCT01878786) A Pilot Study Comparing the Safety and Efficacy of Everolimus With Other Medicines in Recipients of ECD/DCD Kidneys
|
| 46 |
ClinicalTrials.gov (NCT01085097) A Study of Laquinimod in Participants With Systemic Lupus Erythematosus (SLE) Active Lupus Nephritis
|
| 47 |
ClinicalTrials.gov (NCT06068179) Graves' Disease Remission Study: MycoMeth Combo
|
| 48 |
ClinicalTrials.gov (NCT00281879) Donor Stem Cell Transplant or Donor White Blood Cell Infusions in Treating Patients With Hematologic Cancer
|
| 49 |
ClinicalTrials.gov (NCT01659606) Radiation- and Alkylator-free Bone Marrow Transplantation Regimen for Patients With Dyskeratosis Congenita
|
| 50 |
ClinicalTrials.gov (NCT03246906) Comparison of Triple GVHD Prophylaxis Regimens for Nonmyeloablative or Reduced Intensity Conditioning Unrelated Mobilized Blood Cell Transplantation
|
| 51 |
ClinicalTrials.gov (NCT00040846) Alemtuzumab, Fludarabine Phosphate, and Low-Dose Total Body Irradiation Before Donor Stem Cell Transplantation in Treating Patients With Hematological Malignancies
|
| 52 |
ClinicalTrials.gov (NCT00891306) Efficacy and Safety of Human Lipoprotein Lipase (LPL)[S447X] Expressed by an Adeno-Associated Viral Vector in LPL-deficient Subjects
|
| 53 |
ClinicalTrials.gov (NCT05306873) Examining Distinct Immunophenotypes to Validate and Enhance Rational Treatment in Systemic Lupus
|
| 54 |
ClinicalTrials.gov (NCT02370693) Comparing and Combining Bortezomib and Mycophenolate in SSc Pulmonary Fibrosis
|
| 55 |
ClinicalTrials.gov (NCT02949349) Mycophenolate Mofetil Versus Azathioprine for Maintenance Therapy of Lupus Nephritis
|
| 56 |
ClinicalTrials.gov (NCT01856257) Open-Label Phase 2 Trial of a Steroid-Free, CNI-Free, Belatacept-Based Immunosuppressive Regimen
|
| 57 |
ClinicalTrials.gov (NCT00096161) Pentostatin and Lymphocyte Infusion in Preventing Graft Rejection in Patients Who Have Undergone Donor Stem Cell Transplant
|
| 58 |
ClinicalTrials.gov (NCT01951885) Tac, Mini-MTX, MMF Versus Tac, MTX for GVHD Prevention
|
| 59 |
ClinicalTrials.gov (NCT00481819) A Study to Compare the Efficacy and Safety of FK506MR vs Prograf in Patients Undergoing Kidney Transplantation
|
| 60 |
ClinicalTrials.gov (NCT01773616) Trial of Rituximab and Mycophenolate Mofetil Without Oral Steroids for Lupus Nephritis
|
| 61 |
ClinicalTrials.gov (NCT03315052) Budesonide for Immunosuppression After Liver Transplantation to Reduce Side Effects
|
| 62 |
ClinicalTrials.gov (NCT04203108) ATG in HLA-matched Sibling HSCT as GVHD Prophylaxis
|
| 63 |
ClinicalTrials.gov (NCT02342145) Efficacy of Basiliximab in the Prevention of Acute Graft-versus-host Disease in Unrelated Allogeneic Hematopoietic Stem Cell Transplantation Therapy for Thalassemia Major
|
| 64 |
ClinicalTrials.gov (NCT02081755) Safety and Efficacy of Everolimus Treatment in Liver Transplantation for Liver Cancer
|
| 65 |
ClinicalTrials.gov (NCT02077556) Effect of Everolimus on the Pharmacokinetics of Tacrolimus in Renal Transplant Patients
|
| 66 |
ClinicalTrials.gov (NCT00087581) Study of Therapeutic Monitoring of Mycophenolate Mofetil (MMF/CellCept) After Kidney Transplantation
|
| 67 |
ClinicalTrials.gov (NCT00885820) Benefit of Early Protocol Biopsy and Treatment of Subclinical Rejection
|
| 68 |
ClinicalTrials.gov (NCT00650468) A Study to Compare Early Steroid Withdrawal and Long-Term Steroid Maintenance Therapy in Kidney Transplant Patients
|
| 69 |
ClinicalTrials.gov (NCT00118742) Liver Spare the Nephron (STN) Study - A Study of CellCept (Mycophenolate Mofetil) and Sirolimus in Recipients of a Liver Transplant
|
| 70 |
ClinicalTrials.gov (NCT00545402) A Study of CellCept (Mycophenolate Mofetil) in Combination Therapy in Liver Transplant Patients.
|
| 71 |
ClinicalTrials.gov (NCT06178146) Thymosin Alpha-1 for irAE Secondary to ICIs
|
|
|
|
|
|
|