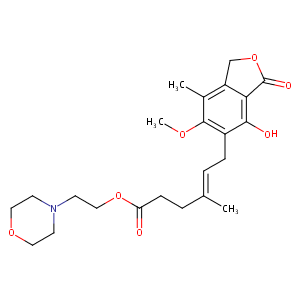
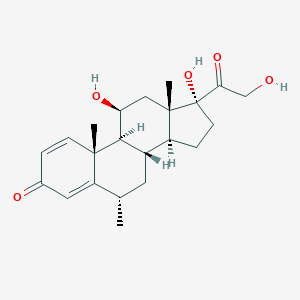
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Cytochrome P450 3A4 (CYP3A4)
|
OTQGYY83
|
CP3A4_HUMAN
|
Decreases Expression
|
[22] |
|
ATP-dependent translocase ABCB1 (ABCB1)
|
OTEJROBO
|
MDR1_HUMAN
|
Increases Expression
|
[23] |
|
Interleukin-6 (IL6)
|
OTUOSCCU
|
IL6_HUMAN
|
Decreases Expression
|
[17] |
|
CCN family member 1 (CCN1)
|
OTKJBEMD
|
CCN1_HUMAN
|
Increases Expression
|
[17] |
|
Phospholipid phosphatase 3 (PLPP3)
|
OTSSF7BK
|
PLPP3_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon-induced protein with tetratricopeptide repeats 3 (IFIT3)
|
OTPGHZB9
|
IFIT3_HUMAN
|
Decreases Expression
|
[17] |
|
Calmegin (CLGN)
|
OTEWVFQV
|
CLGN_HUMAN
|
Decreases Expression
|
[17] |
|
D-3-phosphoglycerate dehydrogenase (PHGDH)
|
OT1LMRTG
|
SERA_HUMAN
|
Decreases Expression
|
[17] |
|
Receptor-interacting serine/threonine-protein kinase 2 (RIPK2)
|
OT0KMIYQ
|
RIPK2_HUMAN
|
Decreases Expression
|
[17] |
|
Gasdermin-E (GSDME)
|
OT1ZWY32
|
GSDME_HUMAN
|
Decreases Expression
|
[17] |
|
Toll-like receptor 2 (TLR2)
|
OTGO5P4R
|
TLR2_HUMAN
|
Decreases Expression
|
[17] |
|
CCN family member 5 (CCN5)
|
OTADU8JJ
|
CCN5_HUMAN
|
Increases Expression
|
[17] |
|
Antiviral innate immune response receptor RIG-I (RIGI)
|
OTFIIC43
|
RIGI_HUMAN
|
Decreases Expression
|
[17] |
|
2'-5'-oligoadenylate synthase 1 (OAS1)
|
OT8ZLOCY
|
OAS1_HUMAN
|
Decreases Expression
|
[17] |
|
Collagen alpha-1(I) chain (COL1A1)
|
OTI31178
|
CO1A1_HUMAN
|
Increases Expression
|
[17] |
|
Superoxide dismutase , mitochondrial (SOD2)
|
OTIWXGZ9
|
SODM_HUMAN
|
Decreases Expression
|
[17] |
|
Ubiquitin-like protein ISG15 (ISG15)
|
OT53QQ7N
|
ISG15_HUMAN
|
Decreases Expression
|
[17] |
|
Thrombospondin-1 (THBS1)
|
OT0ECWK3
|
TSP1_HUMAN
|
Increases Expression
|
[17] |
|
Asparagine synthetase (ASNS)
|
OT8R922G
|
ASNS_HUMAN
|
Decreases Expression
|
[17] |
|
Hepatocyte growth factor receptor (MET)
|
OT7K55MU
|
MET_HUMAN
|
Decreases Expression
|
[17] |
|
Steryl-sulfatase (STS)
|
OTXTJQPC
|
STS_HUMAN
|
Increases Expression
|
[17] |
|
Neuromedin-B (NMB)
|
OT4NLN6H
|
NMB_HUMAN
|
Decreases Expression
|
[17] |
|
Stromelysin-2 (MMP10)
|
OTJDWW8Q
|
MMP10_HUMAN
|
Decreases Expression
|
[17] |
|
Growth-regulated alpha protein (CXCL1)
|
OT3WCTZV
|
GROA_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon alpha-inducible protein 6 (IFI6)
|
OTWOOAM4
|
IFI6_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon-induced protein with tetratricopeptide repeats 1 (IFIT1)
|
OTXOQDSG
|
IFIT1_HUMAN
|
Decreases Expression
|
[17] |
|
Interleukin-8 (CXCL8)
|
OTS7T5VH
|
IL8_HUMAN
|
Decreases Expression
|
[17] |
|
Interleukin-1 receptor antagonist protein (IL1RN)
|
OT308CBE
|
IL1RA_HUMAN
|
Decreases Expression
|
[17] |
|
C-X-C motif chemokine 2 (CXCL2)
|
OTEJCYMY
|
CXCL2_HUMAN
|
Decreases Expression
|
[17] |
|
C-X-C motif chemokine 3 (CXCL3)
|
OTSL94KH
|
CXCL3_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon-induced GTP-binding protein Mx1 (MX1)
|
OT6X8G5T
|
MX1_HUMAN
|
Decreases Expression
|
[17] |
|
Fibulin-1 (FBLN1)
|
OT5MHHOP
|
FBLN1_HUMAN
|
Increases Expression
|
[17] |
|
Atypical chemokine receptor 3 (ACKR3)
|
OTA6GA4F
|
ACKR3_HUMAN
|
Decreases Expression
|
[17] |
|
11-beta-hydroxysteroid dehydrogenase 1 (HSD11B1)
|
OTO7FJA9
|
DHI1_HUMAN
|
Decreases Expression
|
[17] |
|
Adenosine receptor A2a (ADORA2A)
|
OTVRBZ0I
|
AA2AR_HUMAN
|
Decreases Expression
|
[17] |
|
CCN family member 2 (CCN2)
|
OTC39NSU
|
CCN2_HUMAN
|
Increases Expression
|
[17] |
|
Myristoylated alanine-rich C-kinase substrate (MARCKS)
|
OT7N056G
|
MARCS_HUMAN
|
Decreases Expression
|
[17] |
|
Guanylate-binding protein 1 (GBP1)
|
OTUM7RPJ
|
GBP1_HUMAN
|
Decreases Expression
|
[17] |
|
Prostaglandin G/H synthase 2 (PTGS2)
|
OT75U9M4
|
PGH2_HUMAN
|
Decreases Expression
|
[17] |
|
Macrophage metalloelastase (MMP12)
|
OT9Z7LHM
|
MMP12_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon alpha-inducible protein 27, mitochondrial (IFI27)
|
OTI2XGIT
|
IFI27_HUMAN
|
Decreases Expression
|
[17] |
|
Signal transducer and activator of transcription 1-alpha/beta (STAT1)
|
OTLMBUZ6
|
STAT1_HUMAN
|
Decreases Expression
|
[17] |
|
Regulator of G-protein signaling 4 (RGS4)
|
OTG94HHL
|
RGS4_HUMAN
|
Decreases Expression
|
[17] |
|
Prolargin (PRELP)
|
OT9EEBUJ
|
PRELP_HUMAN
|
Increases Expression
|
[17] |
|
Spermine synthase (SMS)
|
OT8JYKNH
|
SPSY_HUMAN
|
Decreases Expression
|
[17] |
|
C-X-C chemokine receptor type 4 (CXCR4)
|
OTUFSBX2
|
CXCR4_HUMAN
|
Decreases Expression
|
[17] |
|
C-C motif chemokine 20 (CCL20)
|
OTUCJY4N
|
CCL20_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon-induced 35 kDa protein (IFI35)
|
OTV9RZ53
|
IN35_HUMAN
|
Decreases Expression
|
[17] |
|
DNA-binding protein inhibitor ID-3 (ID3)
|
OTUULW5Z
|
ID3_HUMAN
|
Increases Expression
|
[17] |
|
Tumor necrosis factor alpha-induced protein 2 (TNFAIP2)
|
OTRZH80H
|
TNAP2_HUMAN
|
Decreases Expression
|
[17] |
|
Aldehyde oxidase (AOX1)
|
OT2FZP6H
|
AOXA_HUMAN
|
Increases Expression
|
[17] |
|
Bone marrow stromal antigen 2 (BST2)
|
OTU4LCJV
|
BST2_HUMAN
|
Decreases Expression
|
[17] |
|
EGF-containing fibulin-like extracellular matrix protein 1 (EFEMP1)
|
OTZVUOOB
|
FBLN3_HUMAN
|
Increases Expression
|
[17] |
|
Neuronal membrane glycoprotein M6-b (GPM6B)
|
OT8Q1582
|
GPM6B_HUMAN
|
Increases Expression
|
[17] |
|
Laminin subunit beta-3 (LAMB3)
|
OTFPU6W8
|
LAMB3_HUMAN
|
Decreases Expression
|
[17] |
|
Nidogen-2 (NID2)
|
OTHC33FF
|
NID2_HUMAN
|
Increases Expression
|
[17] |
|
Signal transducer and activator of transcription 4 (STAT4)
|
OTAK3VFR
|
STAT4_HUMAN
|
Decreases Expression
|
[17] |
|
2'-5'-oligoadenylate synthase-like protein (OASL)
|
OTZ05YRP
|
OASL_HUMAN
|
Decreases Expression
|
[17] |
|
Bcl-2-related protein A1 (BCL2A1)
|
OTWRYSNA
|
B2LA1_HUMAN
|
Decreases Expression
|
[17] |
|
Lymphocyte antigen 6E (LY6E)
|
OTMG16BZ
|
LY6E_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon-induced protein 44-like (IFI44L)
|
OTXKORJP
|
IF44L_HUMAN
|
Decreases Expression
|
[17] |
|
E3 ubiquitin-protein ligase RNF19B (RNF19B)
|
OTYCWKBP
|
RN19B_HUMAN
|
Decreases Expression
|
[17] |
|
Amphoterin-induced protein 2 (AMIGO2)
|
OTPAIT1O
|
AMGO2_HUMAN
|
Decreases Expression
|
[17] |
|
Probable E3 ubiquitin-protein ligase HERC6 (HERC6)
|
OT7G9PQE
|
HERC6_HUMAN
|
Decreases Expression
|
[17] |
|
Equilibrative nucleobase transporter 1 (SLC43A3)
|
OTR60ZLA
|
S43A3_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon-induced protein 44 (IFI44)
|
OTOKSZVA
|
IFI44_HUMAN
|
Decreases Expression
|
[17] |
|
S-adenosylmethionine-dependent nucleotide dehydratase RSAD2 (RSAD2)
|
OTCA9WCM
|
RSAD2_HUMAN
|
Decreases Expression
|
[17] |
|
Nuclear receptor subfamily 4 group A member 3 (NR4A3)
|
OTPBE9R1
|
NR4A3_HUMAN
|
Decreases Expression
|
[17] |
|
Type II iodothyronine deiodinase (DIO2)
|
OTGPNSLH
|
IOD2_HUMAN
|
Increases Expression
|
[17] |
|
Interferon regulatory factor 7 (IRF7)
|
OTC1A2PQ
|
IRF7_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon-stimulated gene 20 kDa protein (ISG20)
|
OTCWRJJW
|
ISG20_HUMAN
|
Decreases Expression
|
[17] |
|
Interferon-induced helicase C domain-containing protein 1 (IFIH1)
|
OTZA2AHA
|
IFIH1_HUMAN
|
Decreases Expression
|
[17] |
|
Anthrax toxin receptor 1 (ANTXR1)
|
OT5W1GPC
|
ANTR1_HUMAN
|
Increases Expression
|
[17] |
|
Interleukin-23 subunit alpha (IL23A)
|
OTYO99HC
|
IL23A_HUMAN
|
Decreases Expression
|
[17] |
|
NADH dehydrogenase 1 alpha subcomplex subunit 4-like 2 (NDUFA4L2)
|
OTK0PG7R
|
NUA4L_HUMAN
|
Decreases Expression
|
[17] |
|
E3 ISG15--protein ligase HERC5 (HERC5)
|
OTZ5PR39
|
HERC5_HUMAN
|
Decreases Expression
|
[17] |
|
Cystine/glutamate transporter (SLC7A11)
|
OTKJ6PXW
|
XCT_HUMAN
|
Decreases Expression
|
[17] |
|
Hypoxia-inducible lipid droplet-associated protein (HILPDA)
|
OTEID3ZM
|
HLPDA_HUMAN
|
Decreases Expression
|
[17] |
|
2'-5'-oligoadenylate synthase 3 (OAS3)
|
OT6E5FYS
|
OAS3_HUMAN
|
Decreases Expression
|
[17] |
|
Coagulation factor VIII (F8)
|
OTH6XHDU
|
FA8_HUMAN
|
Increases Expression
|
[18] |
|
Myelin basic protein (MBP)
|
OTFZCEDB
|
MBP_HUMAN
|
Decreases Expression
|
[24] |
|
Glial fibrillary acidic protein (GFAP)
|
OTQ01ZAS
|
GFAP_HUMAN
|
Decreases Expression
|
[24] |
|
5-hydroxytryptamine receptor 3A (HTR3A)
|
OTAIV1AK
|
5HT3A_HUMAN
|
Decreases Activity
|
[25] |
|
Tyrosine aminotransferase (TAT)
|
OT2CJ91O
|
ATTY_HUMAN
|
Increases ADR
|
[26] |
|
Glucocorticoid receptor (NR3C1)
|
OTCI2YDI
|
GCR_HUMAN
|
Increases ADR
|
[26] |
|
Thrombopoietin (THPO)
|
OTO73DZ2
|
TPO_HUMAN
|
Increases ADR
|
[26] |
|
P-selectin (SELP)
|
OTWR90PK
|
LYAM3_HUMAN
|
Increases ADR
|
[26] |
| ------------------------------------------------------------------------------------ |
|
|
|
|