| 1 |
ClinicalTrials.gov (NCT00420537) Shift to Everolimus (RAD) Kidney Sparing Study
|
| 2 |
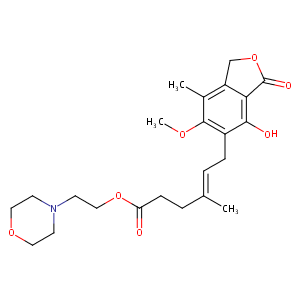
Mycophenolate mofetil FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6831).
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 341).
|
| 5 |
High-Throughput Screening and Identification of Potent Broad-Spectrum Inhibitors of Coronaviruses. J Virol. 2019 May 29;93(12). pii: e00023-19.
|
| 6 |
Clinical and biological impact of ATP-binding cassette transporter activity in adult acute myeloid leukemia. Haematologica. 2023 Jan 1;108(1):61-68.
|
| 7 |
Bronchiolitis obliterans syndrome is associated with increased senescent lymphocytes in the small airways. J Heart Lung Transplant. 2021 Feb;40(2):108-119.
|
| 8 |
Rituximab for rheumatoid arthritis-associated large granular lymphocytic leukemia, a retrospective case series. Semin Arthritis Rheum. 2020 Oct;50(5):1109-1113.
|
| 9 |
Cyclosporin A protects JEG-3 cells against oxidative stress-induced apoptosis by inhibiting the p53 and JNK/p38 signaling pathways. Reprod Biol Endocrinol. 2020 Oct 12;18(1):100.
|
| 10 |
Efficacy and safety of Iguratimod as an add-on therapy for refractory lupus nephritis: A preliminary investigational study. Front Immunol. 2023 Mar 8;14:1062919.
|
| 11 |
Efficacy and safety of extended duration letermovir prophylaxis in recipients of haematopoietic stem-cell transplantation at risk of cytomegalovirus infection: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. 2024 Feb;11(2):e127-e135.
|
| 12 |
Pustular psoriasis. Cutis. 1993 Jan;51(1):29-32.
|
| 13 |
Cyclosporin A (CyA) in primary Sj?gren's syndrome: a double blind study. Ann Rheum Dis. 1986 Sep;45(9):732-5.
|
| 14 |
Why Choose Cyclosporin A as First-line Therapy in COVID-19 Pneumonia. Reumatol Clin. 2020 Apr 16;S1699-258X(20)30044-9.
|
| 15 |
Anti-inflammatory therapy of dry eye. Ocul Surf. 2003 Jan;1(1):31-6.
|
| 16 |
Clinical pipeline report, company report or official report of Roche (2009).
|
| 17 |
Influence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol. 2007 Dec;63(12):1161-9.
|
| 18 |
Influence of drug transporters and UGT polymorphisms on pharmacokinetics of phenolic glucuronide metabolite of mycophenolic acid in Japanese renal transplant recipients. Ther Drug Monit. 2008 Oct;30(5):559-64.
|
| 19 |
PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics. 2014 Jan;24(1):73-9.
|
| 20 |
Characterization of rat intestinal microsomal UDP-glucuronosyltransferase activity toward mycophenolic acid. Drug Metab Dispos. 2006 Sep;34(9):1632-9.
|
| 21 |
The evolution of population pharmacokinetic models to describe the enterohepatic recycling of mycophenolic acid in solid organ transplantation and autoimmune disease. Clin Pharmacokinet. 2011 Jan;50(1):1-24.
|
| 22 |
[Pharmacology of mycophenolate mofetil: recent data and clinical consequences]. Nephrologie. 2001;22(7):331-7.
|
| 23 |
ClinicalTrials.gov (NCT00087581) Study of Therapeutic Monitoring of Mycophenolate Mofetil (MMF/CellCept) After Kidney Transplantation
|
| 24 |
ClinicalTrials.gov (NCT00118742) Liver Spare the Nephron (STN) Study - A Study of CellCept (Mycophenolate Mofetil) and Sirolimus in Recipients of a Liver Transplant
|
| 25 |
ClinicalTrials.gov (NCT01659606) Radiation- and Alkylator-free Bone Marrow Transplantation Regimen for Patients With Dyskeratosis Congenita
|
| 26 |
ClinicalTrials.gov (NCT00040846) Alemtuzumab, Fludarabine Phosphate, and Low-Dose Total Body Irradiation Before Donor Stem Cell Transplantation in Treating Patients With Hematological Malignancies
|
| 27 |
ClinicalTrials.gov (NCT00005799) Fludarabine Phosphate, Low-Dose Total Body Irradiation, and Donor Stem Cell Transplant in Treating Patients With Hematologic Malignancies or Kidney Cancer
|
| 28 |
ClinicalTrials.gov (NCT00014235) Fludarabine Phosphate and Total-Body Radiation Followed by Donor Peripheral Blood Stem Cell Transplant and Immunosuppression in Treating Patients With Hematologic Malignancies
|
| 29 |
ClinicalTrials.gov (NCT00008450) Total-Body Irradiation Followed By Cyclosporine and Mycophenolate Mofetil in Treating Patients With Severe Combined Immunodeficiency Undergoing Donor Bone Marrow Transplant
|
| 30 |
ClinicalTrials.gov (NCT00008177) Radiolabeled Monoclonal Antibody Therapy, Fludarabine Phosphate, and Low-Dose Total-Body Irradiation Followed by Donor Stem Cell Transplant and Immunosuppression Therapy in Treating Older Patients With Advanced Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndromes
|
| 31 |
ClinicalTrials.gov (NCT00005851) Low-Dose Total-Body Irradiation and Fludarabine Phosphate Followed By Donor Peripheral Blood Stem Cell Transplant in Treating Patients With Stage IV Kidney Cancer
|
|
|
|
|
|
|