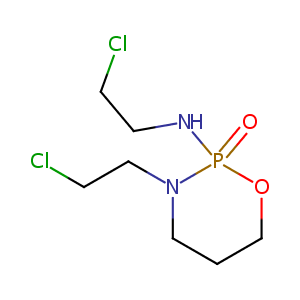
| Synonyms |
Cyfos; Holoxan; Ifex; Ifosfamid; Ifosfamida; Ifosfamidum; Ifosphamide; Ifsofamide; Iphosphamid; Iphosphamide; Isoendoxan; Isofosfamide; Isophosphamide; Isosfamide; Mitoxana; Naxamide; Ifosfamide Sterile; Iso Endoxan; A 4942; ASTA Z 4942; Holoxan 1000; MJF 9325; Z 4942; Z4942; I-Phosphamide; IFEX (TN); Ifex (TN); Ifosfamida [INN-Spanish]; Ifosfamidum [INN-Latin]; Iphosphamid(e); Iso-Endoxan; MJF-9325; Mitoxana (TN); NPFAPI-04; Z-4942; Mitoxana, Ifex, Ifosfamide; Ifosfamide (JAN/USP/INN); Ifosfamide [USAN:INN:BAN:JAN]; N,N-Bis(beta-chloroethyl)-amino-N',O-propylene-phosphoric acid ester diamide; N,3-Bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide; N,3-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylen ephosphoric acid diamide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylene phosphoric acid ester diamide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylenephosphoric acid diamide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylenephosphoric acid ester diamide; N-(2-Chloraethyl)-N'-(2-chloraethyl)-N',O-propylen-phosphorsaureester-diamid; N-(2-Chloraethyl)-N'-(2-chloraethyl)-N',O-propylen-phosphorsaureester-diamid [German]; N-(2-Chloroethyl)-N-(3-(2-chloroethyl)-2-oxido-1,3,2-oxazaphosphinan-2-yl)amine; (+-)-Ifosfamid; (+-)-Ifosphamide; (+-)-Tetrahydro-N,3-bis(2-chloroethyl)-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide; (D,L)-Ifosfamide; (R,S)-Ifosphamide; (R,S)-N,3-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide; 1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide; 1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-, 2-oxide; 2,3-(N,N(sup 1)-Bis(2-chloroethyl)diamido)-1,3,2-oxazaphosphoridinoxyd; 2,3-N,N(sup 1)-Bis(2-chloroethyl)diamido-1,3,2-oxazaphosphoridinoxyd; 2H-1,3,2-Oxazaphosphorin-2-amine, N,3-bis(2-chloroethyl)tetrahydro-, 2-oxide; 2H-1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide; 2H-1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide (8CI); 2H-1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-, 2-oxide; 3,} 2-oxazaphosphorine oxide; 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)perhydro-2H-1,3,2-oxazaphosphorine oxide; 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2H-1,3,2-oxazaphosphorine oxide; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2H-1,3,2-oxazaphosphorineoxide; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide
|