Details of the Drug
General Information of Drug (ID: DMCT3I8)
| Drug Name |
Ifosfamide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cyfos; Holoxan; Ifex; Ifosfamid; Ifosfamida; Ifosfamidum; Ifosphamide; Ifsofamide; Iphosphamid; Iphosphamide; Isoendoxan; Isofosfamide; Isophosphamide; Isosfamide; Mitoxana; Naxamide; Ifosfamide Sterile; Iso Endoxan; A 4942; ASTA Z 4942; Holoxan 1000; MJF 9325; Z 4942; Z4942; I-Phosphamide; IFEX (TN); Ifex (TN); Ifosfamida [INN-Spanish]; Ifosfamidum [INN-Latin]; Iphosphamid(e); Iso-Endoxan; MJF-9325; Mitoxana (TN); NPFAPI-04; Z-4942; Mitoxana, Ifex, Ifosfamide; Ifosfamide (JAN/USP/INN); Ifosfamide [USAN:INN:BAN:JAN]; N,N-Bis(beta-chloroethyl)-amino-N',O-propylene-phosphoric acid ester diamide; N,3-Bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide; N,3-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylen ephosphoric acid diamide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylene phosphoric acid ester diamide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylenephosphoric acid diamide; N-(2-Chloroethyl)-N'-(2-chloroethyl)-N',O-propylenephosphoric acid ester diamide; N-(2-Chloraethyl)-N'-(2-chloraethyl)-N',O-propylen-phosphorsaureester-diamid; N-(2-Chloraethyl)-N'-(2-chloraethyl)-N',O-propylen-phosphorsaureester-diamid [German]; N-(2-Chloroethyl)-N-(3-(2-chloroethyl)-2-oxido-1,3,2-oxazaphosphinan-2-yl)amine; (+-)-Ifosfamid; (+-)-Ifosphamide; (+-)-Tetrahydro-N,3-bis(2-chloroethyl)-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide; (D,L)-Ifosfamide; (R,S)-Ifosphamide; (R,S)-N,3-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide; 1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide; 1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-, 2-oxide; 2,3-(N,N(sup 1)-Bis(2-chloroethyl)diamido)-1,3,2-oxazaphosphoridinoxyd; 2,3-N,N(sup 1)-Bis(2-chloroethyl)diamido-1,3,2-oxazaphosphoridinoxyd; 2H-1,3,2-Oxazaphosphorin-2-amine, N,3-bis(2-chloroethyl)tetrahydro-, 2-oxide; 2H-1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide; 2H-1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide (8CI); 2H-1,3,2-Oxazaphosphorine, 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-, 2-oxide; 3,} 2-oxazaphosphorine oxide; 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)perhydro-2H-1,3,2-oxazaphosphorine oxide; 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2H-1,3,2-oxazaphosphorine oxide; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2H-1,3,2-oxazaphosphorineoxide; 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
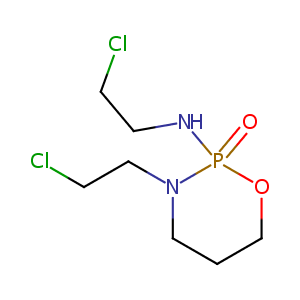 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 261.079 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Ifosfamide
Coadministration of a Drug Treating the Disease Different from Ifosfamide (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
| DIG |
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmaceutical Formulation |
|
||||||||||||||||||||||
References
| 1 | Ifosfamide FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7201). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 8 | Pharmacokinetics and metabolism of ifosfamide in relation to DNA damage assessed by the COMET assay in children with cancer. Br J Cancer. 2005 May 9;92(9):1626-35. | ||||
| 9 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | ||||
| 10 | CYP2C9 polymorphisms in human tumors. Anticancer Res. 2006 Jan-Feb;26(1A):299-305. | ||||
| 11 | Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos. 1999 Jun;27(6):655-66. | ||||
| 12 | Measurement of 4-hydroxylation of ifosfamide in human liver microsomes using the estimation of free and protein-bound acrolein and codetermination of keto- and carboxyifosfamide. J Cancer Res Clin Oncol. 2002 Jul;128(7):385-92. | ||||
| 13 | Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res. 1997 May 15;57(10):1946-54. | ||||
| 14 | A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem. 2003 Apr 18;278(16):14146-52. | ||||
| 15 | Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448. | ||||
| 16 | Transcriptomics hit the target: monitoring of ligand-activated and stress response pathways for chemical testing. Toxicol In Vitro. 2015 Dec 25;30(1 Pt A):7-18. | ||||
| 17 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 18 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 19 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 20 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 21 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 22 | Wong GT, Lee EY, Irwin MG. Contrast induced nephropathy in vascular surgery.?Br J Anaesth. 2016;117 Suppl 2:ii63-ii73. [PMID: 27566809] | ||||
| 23 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 24 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 25 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 26 | Product Information. Agenerase (amprenavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 27 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 28 | Product Information. Arava (leflunomide). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 29 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 30 | Product Information. Prolia (denosumab). Amgen USA, Thousand Oaks, CA. | ||||
| 31 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 32 | Product Information. Vumerity (diroximel fumarate). Alkermes, Inc, Cambridge, MA. | ||||
| 33 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 35 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 36 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 37 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 38 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 39 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 40 | Product Information. Arcalyst (rilonacept). Regeneron Pharmaceuticals Inc, Tarrytown, NY. | ||||
| 41 | Product Information. Cimzia (certolizumab). UCB Pharma Inc, Smyrna, GA. | ||||
| 42 | CDC. Centers for Disease Control and Prevention/ "Recommendations of the advisory committtee on immunization practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence." MMWR Morb Mortal Wkly Rep 42(RR-04) (1993): 1-18. [PMID: 20300058] | ||||
| 43 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
