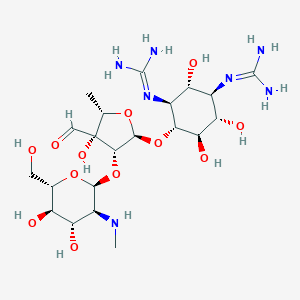
| Synonyms |
Agrept; Agrimycin; Gerox; Neodiestreptopab; SRY; Strepcen; Streptomicina; Streptomycine; Streptomycinum; Streptomyzin; Liposomal Streptomycin; Streptomicina [Italian]; Streptomycin A; Streptomycin A sulfate; Streptomycin Sesquisulfate Hydrate; Streptomycin sulfate; Streptomycin sulphate; Streptomyzin [German]; Agrept (TN); Estreptomicina [INN-Spanish]; Hokko-mycin; Plantomycin (TN); Rimosidin (TN); Streptomycin & EEP; Streptomycin & Propolis; Streptomycin (INN); Streptomycin (TN); Streptomycin [INN:BAN]; Streptomycin, Sulfate Salt; AS-50 (TN); STREPTOMYCIN SULFATE (2:3) SALT; Agri-mycin-17 (TN); O-2-Deoxy-2-(methylamino)-.alpha.-L-glucopyranosyl-(1->2)-O-5-deoxy-3-C-formyl-.alpha.-L-lyxofuranosyl-(1->4)-N,N'-bis(aminoiminomethyl)-D-streptamine and Liposome; N,N'''-[(1R,2R,3S,4R,5R,6S)-4-{5-deoxy-2-O-[2-deoxy-2-(methylamino)-alpha-L-glucopyranosyl]-3-C-formyl-alpha-L-lyxofuranosyloxy}-2,5,6-trihydroxycyclohexane-1,3-diyl]diguanidine; N,N'''-[(1R,2R,3S,4R,5R,6S)-4-({5-deoxy-2-O-[2-deoxy-2-(methylamino)-alpha-L-glucopyranosyl]-3-C-formyl-alpha-L-lyxofuranosyl}oxy)-2,5,6-trihydroxycyclohexane-1,3-diyl]diguanidine; 2,4-Diguanidino-3,5,6-trihydroxycyclohexyl 5-deoxy-2-O-(2-deoxy-2-methylamino-alpha-L-glucopyranosyl)-3-C-formyl-beta-L-lyxopentanofuranoside; 2-[(1R,2R,3S,4R,5R,6S)-3-(diaminomethylideneamino)-4-[(2R,3R,4R,5S)-3-[(2S,3S,4S,5R,6S)-4,5-dihydroxy-6-(hydroxymethyl)-3-(methylamino)oxan-2-yl]oxy-4-formyl-4-hydroxy-5-methyloxolan-2-yl]oxy-2,5,6-trihydroxycyclohexyl]guanidine; 2-[(1R,2R,3S,4R,5R,6S)-3-(diaminomethylideneamino)-4-[(2S,3S,4S,5R)-3-[(2R,3R,4R,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-(methylamino)oxan-2-yl]oxy-4-formyl-4-hydroxy-5-methyloxolan-2-yl]oxy-2,5,6-trihydroxycyclohexyl]guanidine; 2-[(1S,2S,3R,4S,5S,6R)-3-(diaminomethylideneamino)-4-[(2R,3R,4R,5S)-3-[(2S,3S,4S,5R,6S)-4,5-dihydroxy-6-(hydroxymethyl)-3-(methylamino)oxan-2-yl]oxy-4-formyl-4-hydroxy-5-methyloxolan-2-yl]oxy-2,5,6-trihydroxycyclohexyl]guanidine; 2-[(1S,4S)-5-(diaminomethylideneamino)-2-[(2R,5S)-3-[(2S,5R)-4,5-dihydroxy-6-(hydroxymethyl)-3-(methylamino)oxan-2-yl]oxy-4-formyl-4-hydroxy-5-methyloxolan-2-yl]oxy-3,4,6-trihydroxycyclohexyl]guanidine; [2-deoxy-2-(dimethylamino)-alpha-L-glucopyranosyl]-(1->2)-[5-deoxy-3-C-formyl-alpha-L-lyxofuranosyl]-(1->4)-{N',N'''-[(1,3,5/2,4,6)-2,4,5,6-tetrahydroxycyclohexane-1,3-diyl]diguanidine}
|