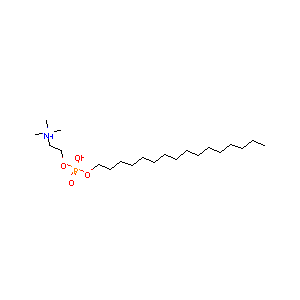
| Synonyms |
HDPC; HePC; Hexadecylphosphocholine; Hexadecylphosphorylcholine; Impavido; Miltefosina; Miltefosinum; Miltex; Baxter Oncology brand of miltefosine; Baxter brand of miltefosine; Miltefosin C; Prasfarma brand of miltefosine; D 18506; IN1227; D-18506; H-1850; M-7200; Miltefosina [INN-Spanish]; Miltefosine (INN); Miltefosine[INN:BAN]; Miltefosinum [INN-Latin]; N-Hexadecylphosphorylcholine; TF-002; Choline hydroxide, hexadecyl hydrogen phosphate, inner salt; Choline, hexadecyl hydrogen phosphate, inner salt; Hexadecyl 2-(trimethylazaniumyl)ethyl phosphate; Choline phosphate, hexadecyl ester, hydroxide, inner salt; Choline phosphate, hexadecyl ester, hydroxide, inner salt (6CI); Hexadecyl 2-(trimethyl-.lambda.~5~-azanyl)ethyl hydrogen phosphate; Ethanaminium, 2-(((hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-trimethyl-, hydroxide, inner salt; Ethanaminium, 2-(((hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-trimethyl-, hydroxide, innner salt; 1-Hexadecylphosphorylcholine; 2-(((Hexadecyloxy)hydroxyphosphinyl)oxy)-N,N,N-trimethylethanaminium hydroxide, inner salt; 3,5-Dioxa-4-phosphaunacosan-1-aminium, 4-hydroxy-N,N,N-trimethyl-, hydroxide, inner salt, 4-oxide
|