| Synonyms |
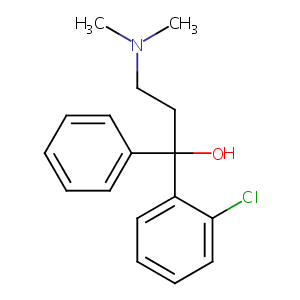
Abehol; Antitussin; Calmotusin; Chlofedanol; Clofedano; Clofedanol; Clofedanolum; Clofedianolo; Dencyl; Eutus; Tussistop; Clofedianolo [Italian]; Clophedianol base; Ulo base; SL 501 base; Antitussin (TN); Clofedanol (INN); Clofedanol [INN:BAN]; Clofedanolum [INN-Latin]; Ulone (TN); Alpha-(Dimethylaminoethyl)-o-chlorobenzhydrol; Benzenemethanol, 2-chloro-alpha-(2-(dimethylamino)ethyl)-alpha-phenyl-(9CI); 1-(2-Chlorophenyl)-1-phenyl-3-dimethylaminopropanol; 1-(2-Chlorophenyl)-3-(dimethylamino)-1-phenyl-1-propanol; 1-(2-chlorophenyl)-3-(dimethylamino)-1-phenylpropan-1-ol; 1-(2-chlorophenyl)-3-dimethylamino-1-phenylpropan-1-ol; 1-Phenyl-1-(o-chlorophenyl)-3-dimethylaminopropanol; 2-Chloro-.alpha.-(2-dimethylaminoethyl)benzhydrol; 2-Chloro-alpha-(2-(dimethylamino)ethyl)benzhydrol; 2-Cloro-alpha-(2-dimetilaminoetil)-benzidrolo; 2-Cloro-alpha-(2-dimetilaminoetil)-benzidrolo [Italian]
|