Details of the Drug
General Information of Drug (ID: DMXGJEI)
| Drug Name |
Chlophedianol
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Abehol; Antitussin; Calmotusin; Chlofedanol; Clofedano; Clofedanol; Clofedanolum; Clofedianolo; Dencyl; Eutus; Tussistop; Clofedianolo [Italian]; Clophedianol base; Ulo base; SL 501 base; Antitussin (TN); Clofedanol (INN); Clofedanol [INN:BAN]; Clofedanolum [INN-Latin]; Ulone (TN); Alpha-(Dimethylaminoethyl)-o-chlorobenzhydrol; Benzenemethanol, 2-chloro-alpha-(2-(dimethylamino)ethyl)-alpha-phenyl-(9CI); 1-(2-Chlorophenyl)-1-phenyl-3-dimethylaminopropanol; 1-(2-Chlorophenyl)-3-(dimethylamino)-1-phenyl-1-propanol; 1-(2-chlorophenyl)-3-(dimethylamino)-1-phenylpropan-1-ol; 1-(2-chlorophenyl)-3-dimethylamino-1-phenylpropan-1-ol; 1-Phenyl-1-(o-chlorophenyl)-3-dimethylaminopropanol; 2-Chloro-.alpha.-(2-dimethylaminoethyl)benzhydrol; 2-Chloro-alpha-(2-(dimethylamino)ethyl)benzhydrol; 2-Cloro-alpha-(2-dimetilaminoetil)-benzidrolo; 2-Cloro-alpha-(2-dimetilaminoetil)-benzidrolo [Italian]
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anesthetics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
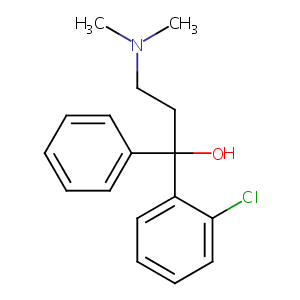 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 289.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Chlophedianol (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||
References
