Details of the Drug
General Information of Drug (ID: DM1I8LU)
| Drug Name |
Coenzyme A
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
coenzyme A; CoASH; CoA-SH; Zeel; 85-61-0; Depot-Zeel; CoA; HSCoA; Coenzym A; Coenzyme ASH; co-enzyme-A; UNII-SAA04E81UX; HS-CoA; SAA04E81UX; CHEBI:15346; 3'-phosphoadenosine-(5')diphospho(4')pantatheine; Coenzyme A hydrate; co-A; Co-A-SH; Reduced CoA; 3'-phosphoadenosine 5'-{3-[(3R)-3-hydroxy-2,2-dimethyl-4-oxo-4-({3-oxo-3-[(2-sulfanylethyl)amino]propyl}amino)butyl] dihydrogen diphosphate}
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
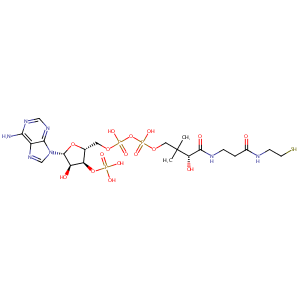 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 767.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -5.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 18 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 21 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
References
